The G2 checkpoint-a node-based molecular switch
- PMID: 28396830
- PMCID: PMC5377395
- DOI: 10.1002/2211-5463.12206
The G2 checkpoint-a node-based molecular switch
Abstract
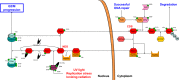
Tight regulation of the eukaryotic cell cycle is paramount to ensure genomic integrity throughout life. Cell cycle checkpoints are present in each phase of the cell cycle and prevent cell cycle progression when genomic integrity is compromised. The G2 checkpoint is an intricate signaling network that regulates the progression of G2 to mitosis (M). We propose here a node-based model of G2 checkpoint regulation, in which the action of the central CDK1-cyclin B1 node is determined by the concerted but opposing activities of the Wee1 and cell division control protein 25C (CDC25C) nodes. Phosphorylation of both Wee1 and CDC25C at specific sites determines their subcellular localization, driving them either toward activity within the nucleus or to the cytoplasm and subsequent ubiquitin-mediated proteasomal degradation. In turn, this subcellular balance of the Wee1 and CDC25C nodes is directed by the action of the PLK1 and CHK1 nodes via what we have termed the 'nuclear and cytoplasmic decision states' of Wee1 and CDC25C. The proposed node-based model provides an intelligible structure of the complex interactions that govern the decision to delay or continue G2/M progression. The model may also aid in predicting the effects of agents that target these G2 checkpoint nodes.
Keywords: CDC25C; CHK1; G2 checkpoint; PLK1; Wee1; cell cycle.
Figures







References
-
- Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274, 1664–1672. - PubMed
-
- Medema RH and Macurek L (2012) Checkpoint control and cancer. Oncogene 31, 2601–2613. - PubMed
-
- Malumbres M and Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9, 153–166. - PubMed
-
- Stewart M (2007) Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol 8, 195–208. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

