Proteome Profiles of Digested Products of Commercial Meat Sources
- PMID: 28396857
- PMCID: PMC5366984
- DOI: 10.3389/fnut.2017.00008
Proteome Profiles of Digested Products of Commercial Meat Sources
Abstract
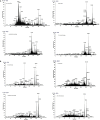
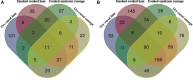
This study was designed to characterize in vitro-digested products of proteins from four commercial meat products, including dry-cured ham, cooked ham, emulsion-type sausage, and dry-cured sausage. The samples were homogenized and incubated with pepsin and trypsin. The digestibility and particle sizes of digested products were measured. Nano-LC-MS/MS was applied to characterize peptides. The results showed the highest digestibility and the lowest particle size in dry-cured ham (P < 0.05), while the opposite was for cooked ham (P < 0.05). Nano-LC-MS/MS analysis revealed that dry-cured ham samples had the greatest number of 750-3,500 Da Mw peptides in pepsin-digested products. In the digested products of cooked ham and emulsion-type sausage, a lot of peptides were matched with soy protein that was added in the formulations. In addition, protein oxidation was also observed in different meat products. Our findings give an insight into nutritional values of different meat products.
Keywords: LC–MS/MS; digestibility; in vitro digestion; meat products; oxidation; particle size.
Figures


Similar articles
-
In vitro protein digestibility of pork products is affected by the method of processing.Food Res Int. 2017 Feb;92:88-94. doi: 10.1016/j.foodres.2016.12.024. Epub 2016 Dec 28. Food Res Int. 2017. PMID: 28290301
-
Changes in the extent and products of In vitro protein digestion during the ripening periods of Chinese dry-cured hams.Meat Sci. 2021 Jan;171:108290. doi: 10.1016/j.meatsci.2020.108290. Epub 2020 Aug 26. Meat Sci. 2021. PMID: 32949821
-
In vitro protein digestion of pork cuts differ with muscle type.Food Res Int. 2018 Apr;106:344-353. doi: 10.1016/j.foodres.2017.12.070. Epub 2017 Dec 28. Food Res Int. 2018. PMID: 29579934
-
Perspectives in the Use of Peptidomics in Ham.Proteomics. 2018 Sep;18(18):e1700422. doi: 10.1002/pmic.201700422. Epub 2018 Jun 27. Proteomics. 2018. PMID: 29882253 Review.
-
Understanding the implications of current health trends on the aroma of wet and dry cured meat products.Meat Sci. 2018 Oct;144:53-61. doi: 10.1016/j.meatsci.2018.04.016. Epub 2018 Apr 20. Meat Sci. 2018. PMID: 29716761 Review.
Cited by
-
Stability of Antiradical Activity of Protein Extracts and Hydrolysates from Dry-Cured Pork Loins with Probiotic Strains of LAB.Nutrients. 2018 Apr 22;10(4):521. doi: 10.3390/nu10040521. Nutrients. 2018. PMID: 29690547 Free PMC article.
-
Effects of bromelain on the quality of smoked salted duck.Food Sci Nutr. 2021 Jun 24;9(8):4473-4483. doi: 10.1002/fsn3.2422. eCollection 2021 Aug. Food Sci Nutr. 2021. PMID: 34401095 Free PMC article.
References
-
- Remond D, Savary-Auzeloux I, Gatellier P, Sante-Lhoutellier V. Nutritional properties of meat peptides and proteins: impact of processing. Sci Des Aliments (2008) 28:389–401.10.3166/sda.28.389-401 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

