Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: comparison with secondary progressive multiple sclerosis
- PMID: 28396953
- PMCID: PMC5489620
- DOI: 10.1007/s10072-017-2933-6
Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: comparison with secondary progressive multiple sclerosis
Abstract
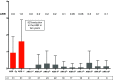
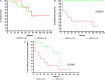
The main objective of our work is to describe the long-term results of myeloablative autologous hematopoietic stem cell transplant (AHSCT) in multiple sclerosis patients. Patients that failed to conventional therapies for multiple sclerosis (MS) underwent an approved protocol for AHSCT, which consisted of peripheral blood stem cell mobilization with cyclophosphamide and granulocyte colony-stimulating factor (G-CSF), followed by a conditioning regimen of BCNU, Etoposide, Ara-C, Melphalan IV, plus Rabbit Thymoglobulin. Thirty-eight MS patients have been transplanted since 1999. Thirty-one patients have been followed for more than 2 years (mean 8.4 years). There were 22 relapsing-remitting multiple sclerosis (RRMS) patients and 9 secondary progressive multiple sclerosis (SPMS) patients. No death related to AHSCT. A total of 10 patients (32.3%) had at least one relapse during post-AHSCT evolution, 6 patients in the RRMS group (27.2%) and 4 in the SPMS group (44.4%). After AHSCT, 7 patients (22.6%) experienced progression of disability, all within SP form. By contrast, no patients with RRMS experienced worsening of disability after a median follow-up of 5.4 years, 60% of them showed a sustained reduction in disability (SRD), defined as the improvement of 1.0 point in the expanded disability status scale (EDSS) sustains for 6 months (0.5 in cases of EDSS ≥ 5.5). The only clinical variable that predicted a poor response to AHSCT was a high EDSS in the year before transplant. AHSCT using the BEAM-ATG scheme is safe and efficacious to control the aggressive forms of RRMS.
Keywords: Autologous hematopoietic stem cell transplantation; Immunosupression; Immunotherapy; Multiple sclerosis; Neurodegeneration; Secondary progressive multiple sclerosis.
Conflict of interest statement
The local Ethics Committee of Clinical Investigation of the University Hospital La Fe and University Clinic Hospital of València approved the treatment protocol. Each patient was selected at the Neuroimmunology Units of the participant Centers and signed the informed consent.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

