Secukinumab is Efficacious and Safe in Hispanic Patients with Moderate-to-Severe Plaque Psoriasis: Pooled Analysis of Four Phase 3 Trials
- PMID: 28397079
- PMCID: PMC5487875
- DOI: 10.1007/s12325-017-0521-z
Secukinumab is Efficacious and Safe in Hispanic Patients with Moderate-to-Severe Plaque Psoriasis: Pooled Analysis of Four Phase 3 Trials
Erratum in
-
Erratum to: Secukinumab is Efficacious and Safe in Hispanic Patients with Moderate-to-Severe Plaque Psoriasis: Pooled Analysis of Four Phase 3 Trials.Adv Ther. 2017 Jul;34(7):1772. doi: 10.1007/s12325-017-0563-2. Adv Ther. 2017. PMID: 28589404 Free PMC article. No abstract available.
Abstract
Introduction: There is little evidence available on the efficacy and safety of biologic therapies for the treatment of psoriasis in Hispanic patients. Secukinumab is demonstrated to be highly effective for clearing psoriasis. The aim of this study was to compare the efficacy and safety of secukinumab in Hispanic and non-Hispanic patients.
Methods: Data were pooled from four phase 3 studies of secukinumab in patients with moderate-to-severe plaque psoriasis. Patients who self-identified as Hispanic were included in the Hispanic subgroup.
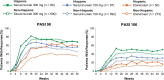
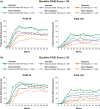
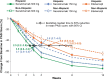
Results: Efficacy responses (Psoriasis Area and Severity Index [PASI] 75/90/100 and Investigator's Global Assessment 2011 modified version 0/1) for secukinumab 300 mg were greater than for etanercept at week 12 in the Hispanic and non-Hispanic patient subgroups. At week 12 with secukinumab 300 mg, PASI 90/100 responses were achieved by 70.6%/35.9% of Hispanic patients and 58.0%/28.1% of non-Hispanic patients. At week 12 with secukinumab 150 mg, PASI 90/100 responses were achieved by 59.5%/25.1% of Hispanic patients and 41.2%/13.4% of non-Hispanic patients. In both subgroups, peak efficacy responses with secukinumab were observed at week 16 and were maintained to week 52.
Conclusions: Secukinumab is highly effective for clearing psoriasis in both Hispanic and non-Hispanic patients.
Funding: Novartis Pharmaceutical Corporation.
Keywords: Biologic therapy; Dermatology Life Quality Index; Hispanic; IL-17; Moderate-to-severe psoriasis; Non-Hispanic; Pooled analysis; Psoriasis Area and Severity Index; Secukinumab; Subcutaneous injection.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

