Co-option of an endogenous retrovirus envelope for host defense in hominid ancestors
- PMID: 28397686
- PMCID: PMC5388530
- DOI: 10.7554/eLife.22519
Co-option of an endogenous retrovirus envelope for host defense in hominid ancestors
Abstract
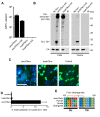
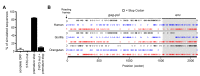
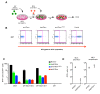
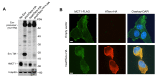
Endogenous retroviral sequences provide a molecular fossil record of ancient infections whose analysis might illuminate mechanisms of viral extinction. A close relative of gammaretroviruses, HERV-T, circulated in primates for ~25 million years (MY) before apparent extinction within the past ~8 MY. Construction of a near-complete catalog of HERV-T fossils in primate genomes allowed us to estimate a ~32 MY old ancestral sequence and reconstruct a functional envelope protein (ancHTenv) that could support infection of a pseudotyped modern gammaretrovirus. Using ancHTenv, we identify monocarboxylate transporter-1 (MCT-1) as a receptor used by HERV-T for attachment and infection. A single HERV-T provirus in hominid genomes includes an env gene (hsaHTenv) that has been uniquely preserved. This apparently exapted HERV-T env could not support virion infection but could block ancHTenv mediated infection, by causing MCT-1 depletion from cell surfaces. Thus, hsaHTenv may have contributed to HERV-T extinction, and could also potentially regulate cellular metabolism.
Keywords: Endogenous retrovirus; Hominid; Paleovirology; evolutionary biology; genomics; human; infectious disease; microbiology; receptors; virus.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures









Comment in
-
Pushing the envelope.Elife. 2017 Apr 13;6:e26397. doi: 10.7554/eLife.26397. Elife. 2017. PMID: 28406395 Free PMC article.
Similar articles
-
Identification of the Receptor Used by the Ecotropic Mouse GLN Endogenous Retrovirus.J Virol. 2019 Feb 19;93(5):e01125-18. doi: 10.1128/JVI.01125-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541852 Free PMC article.
-
Infectious Entry Pathway Mediated by the Human Endogenous Retrovirus K Envelope Protein.J Virol. 2016 Jan 20;90(7):3640-9. doi: 10.1128/JVI.03136-15. J Virol. 2016. PMID: 26792739 Free PMC article.
-
FeLIX is a restriction factor for mammalian retrovirus infection.J Virol. 2024 Apr 16;98(4):e0177123. doi: 10.1128/jvi.01771-23. Epub 2024 Mar 5. J Virol. 2024. PMID: 38440982 Free PMC article.
-
Expression and regulation of human endogenous retrovirus W elements.APMIS. 2016 Jan-Feb;124(1-2):52-66. doi: 10.1111/apm.12478. APMIS. 2016. PMID: 26818262 Review.
-
Paleovirology of 'syncytins', retroviral env genes exapted for a role in placentation.Philos Trans R Soc Lond B Biol Sci. 2013 Aug 12;368(1626):20120507. doi: 10.1098/rstb.2012.0507. Print 2013 Sep 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23938756 Free PMC article. Review.
Cited by
-
Co-option of the lineage-specific LAVA retrotransposon in the gibbon genome.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19328-19338. doi: 10.1073/pnas.2006038117. Epub 2020 Jul 20. Proc Natl Acad Sci U S A. 2020. PMID: 32690705 Free PMC article.
-
Widespread expression of the ancient HERV-K (HML-2) provirus group in normal human tissues.PLoS Biol. 2022 Oct 18;20(10):e3001826. doi: 10.1371/journal.pbio.3001826. eCollection 2022 Oct. PLoS Biol. 2022. PMID: 36256614 Free PMC article.
-
Crosstalk between the Intestinal Virome and Other Components of the Microbiota, and Its Effect on Intestinal Mucosal Response and Diseases.J Immunol Res. 2022 Sep 27;2022:7883945. doi: 10.1155/2022/7883945. eCollection 2022. J Immunol Res. 2022. PMID: 36203793 Free PMC article. Review.
-
Evolution and antiviral activity of a human protein of retroviral origin.Science. 2022 Oct 28;378(6618):422-428. doi: 10.1126/science.abq7871. Epub 2022 Oct 27. Science. 2022. PMID: 36302021 Free PMC article.
-
Reduced Folate Carrier: an Entry Receptor for a Novel Feline Leukemia Virus Variant.J Virol. 2019 Jun 14;93(13):e00269-19. doi: 10.1128/JVI.00269-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996094 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

