Non-Anticoagulant Heparins Are Hepcidin Antagonists for the Treatment of Anemia
- PMID: 28397746
- PMCID: PMC6154463
- DOI: 10.3390/molecules22040598
Non-Anticoagulant Heparins Are Hepcidin Antagonists for the Treatment of Anemia
Abstract
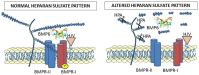
The peptide hormone hepcidin is a key controller of systemic iron homeostasis, and its expression in the liver is mainly regulated by bone morphogenetic proteins (BMPs), which are heparin binding proteins. In fact, heparins are strong suppressors of hepcidin expression in hepatic cell lines that act by inhibiting the phosphorylation of SMAD1/5/8 proteins elicited by the BMPs. The inhibitory effect of heparins has been demonstrated in cells and in mice, where subcutaneous injections of non-anticoagulant heparins inhibited liver hepcidin expression and increased iron bioavailability. The chemical characteristics for high anti-hepcidin activity in vitro and in vivo include the 2O-and 6O-sulfation and a molecular weight above 7 kDa. The most potent heparins have been found to be the super-sulfated ones, active in hepcidin suppression with a molecular weight as low as 4 kDa. Moreover, the alteration of endogenous heparan sulfates has been found to cause a reduction in hepcidin expression in vitro and in vivo, indicating that heparins act by interfering with the interaction between BMPs and components of the complex involved in the activation of the BMP/SMAD1/5/8 pathway. This review summarizes recent findings on the anti-hepcidin activity of heparins and their possible use for the treatment of anemia caused by hepcidin excess, including the anemia of chronic diseases.
Keywords: anemia; heparin; hepcidin; iron homeostasis.
Conflict of interest statement
The authors declare non conflict of interest.
Figures

Similar articles
-
Low anticoagulant heparin-iron complex targeting inhibition of hepcidin ameliorates anemia of chronic disease in rodents.Eur J Pharmacol. 2021 Apr 15;897:173958. doi: 10.1016/j.ejphar.2021.173958. Epub 2021 Feb 19. Eur J Pharmacol. 2021. PMID: 33610598
-
Glycol-split nonanticoagulant heparins are inhibitors of hepcidin expression in vitro and in vivo.Blood. 2014 Mar 6;123(10):1564-73. doi: 10.1182/blood-2013-07-515221. Epub 2014 Jan 7. Blood. 2014. PMID: 24398330 Free PMC article.
-
Oversulfated heparins with low anticoagulant activity are strong and fast inhibitors of hepcidin expression in vitro and in vivo.Biochem Pharmacol. 2014 Dec 1;92(3):467-75. doi: 10.1016/j.bcp.2014.09.007. Epub 2014 Sep 21. Biochem Pharmacol. 2014. PMID: 25241290
-
The role of heparin, heparanase and heparan sulfates in hepcidin regulation.Vitam Horm. 2019;110:157-188. doi: 10.1016/bs.vh.2019.01.008. Epub 2019 Feb 11. Vitam Horm. 2019. PMID: 30798810 Review.
-
Hepcidin.Adv Chronic Kidney Dis. 2019 Jul;26(4):298-305. doi: 10.1053/j.ackd.2019.04.005. Adv Chronic Kidney Dis. 2019. PMID: 31477260 Review.
Cited by
-
Looking Forward to the Future of Heparin: New Sources, Developments and Applications.Molecules. 2018 Jan 31;23(2):293. doi: 10.3390/molecules23020293. Molecules. 2018. PMID: 29385025 Free PMC article.
-
Retinal Degeneration and Alzheimer's Disease: An Evolving Link.Int J Mol Sci. 2020 Oct 2;21(19):7290. doi: 10.3390/ijms21197290. Int J Mol Sci. 2020. PMID: 33023198 Free PMC article. Review.
-
Potential Use of Anti-Inflammatory Synthetic Heparan Sulfate to Attenuate Liver Damage.Biomedicines. 2020 Nov 16;8(11):503. doi: 10.3390/biomedicines8110503. Biomedicines. 2020. PMID: 33207634 Free PMC article. Review.
-
Local synthesis of hepcidin in the anterior segment of the eye: A novel observation with physiological and pathological implications.Exp Eye Res. 2020 Jan;190:107890. doi: 10.1016/j.exer.2019.107890. Epub 2019 Dec 4. Exp Eye Res. 2020. PMID: 31811823 Free PMC article.
-
Iron metabolism and management: focus on chronic kidney disease.Kidney Int Suppl (2011). 2021 Apr;11(1):46-58. doi: 10.1016/j.kisu.2020.12.003. Epub 2021 Mar 18. Kidney Int Suppl (2011). 2021. PMID: 33777495 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

