Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology
- PMID: 28397763
- PMCID: PMC5412376
- DOI: 10.3390/ijms18040792
Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology
Abstract
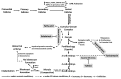
The endoplasmic reticulum (ER), comprises 60% of the total cell membrane and interacts directly or indirectly with several cell organelles i.e., Golgi bodies, mitochondria and proteasomes. The ER is usually associated with large numbers of attached ribosomes. During evolution, ER developed as the specific cellular site of synthesis, folding, modification and trafficking of secretory and cell-surface proteins. The ER is also the major intracellular calcium storage compartment that maintains cellular calcium homeostasis. During the production of functionally effective proteins, several ER-specific molecular steps sense quantity and quality of synthesized proteins as well as proper folding into their native structures. During this process, excess accumulation of unfolded/misfolded proteins in the ER lumen results in ER stress, the homeostatic coping mechanism that activates an ER-specific adaptation program, (the unfolded protein response; UPR) to increase ER-associated degradation of structurally and/or functionally defective proteins, thus sustaining ER homeostasis. Impaired ER homeostasis results in aberrant cellular responses, contributing to the pathogenesis of various diseases. Both female and male reproductive tissues undergo highly dynamic cellular, molecular and genetic changes such as oogenesis and spermatogenesis starting in prenatal life, mainly controlled by sex-steroids but also cytokines and growth factors throughout reproductive life. These reproductive changes require ER to provide extensive protein synthesis, folding, maturation and then their trafficking to appropriate cellular location as well as destroying unfolded/misfolded proteins via activating ER-associated degradation mediated proteasomes. Many studies have now shown roles for ER stress/UPR signaling cascades in the endometrial menstrual cycle, ovarian folliculogenesis and oocyte maturation, spermatogenesis, fertilization, pre-implantation embryo development and pregnancy and parturition. Conversely, the contribution of impaired ER homeostasis by severe/prolong ER stress-mediated UPR signaling pathways to several reproductive tissue pathologies including endometriosis, cancers, recurrent pregnancy loss and pregnancy complications associated with pre-term birth have been reported. This review focuses on ER stress and UPR signaling mechanisms, and their potential roles in female and male reproductive physiopathology involving in menstrual cycle changes, gametogenesis, preimplantation embryo development, implantation and placentation, labor, endometriosis, pregnancy complications and preterm birth as well as reproductive system tumorigenesis.
Keywords: decidua; endoplasmic reticulum stress; ovary; placenta; testes; uterus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Simonyi I., Pataki S., Kalman K., Buda L. Determination of the active ingredient content in Tavegyl tablets. Acta Pharm. Hung. 1975;45:237–244. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

