Small and Random Peptides: An Unexplored Reservoir of Potentially Functional Primitive Organocatalysts. The Case of Seryl-Histidine
- PMID: 28397774
- PMCID: PMC5492141
- DOI: 10.3390/life7020019
Small and Random Peptides: An Unexplored Reservoir of Potentially Functional Primitive Organocatalysts. The Case of Seryl-Histidine
Abstract
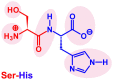
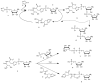
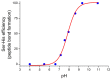
Catalysis is an essential feature of living systems biochemistry, and probably, it played a key role in primordial times, helping to produce more complex molecules from simple ones. However, enzymes, the biocatalysts par excellence, were not available in such an ancient context, and so, instead, small molecule catalysis (organocatalysis) may have occurred. The best candidates for the role of primitive organocatalysts are amino acids and short random peptides, which are believed to have been available in an early period on Earth. In this review, we discuss the occurrence of primordial organocatalysts in the form of peptides, in particular commenting on reports about seryl-histidine dipeptide, which have recently been investigated. Starting from this specific case, we also mention a peptide fragment condensation scenario, as well as other potential roles of peptides in primordial times. The review actually aims to stimulate further investigation on an unexplored field of research, namely one that specifically looks at the catalytic activity of small random peptides with respect to reactions relevant to prebiotic chemistry and early chemical evolution.
Keywords: Ser-His; fragment condensation; organocatalysis; peptide bond formation; phosphodiester bond formation; small peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

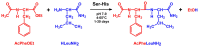




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

