The Transmembrane Morphogenesis Protein gp1 of Filamentous Phages Contains Walker A and Walker B Motifs Essential for Phage Assembly
- PMID: 28397779
- PMCID: PMC5408679
- DOI: 10.3390/v9040073
The Transmembrane Morphogenesis Protein gp1 of Filamentous Phages Contains Walker A and Walker B Motifs Essential for Phage Assembly
Abstract
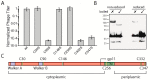
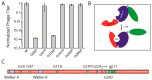
In contrast to lytic phages, filamentous phages are assembled in the inner membrane and secreted across the bacterial envelope without killing the host. For assembly and extrusion of the phage across the host cell wall, filamentous phages code for membrane-embedded morphogenesis proteins. In the outer membrane of Escherichia coli, the protein gp4 forms a pore-like structure, while gp1 and gp11 form a complex in the inner membrane of the host. By comparing sequences with other filamentous phages, we identified putative Walker A and B motifs in gp1 with a conserved lysine in the Walker A motif (K14), and a glutamic and aspartic acid in the Walker B motif (D88, E89). In this work we demonstrate that both, Walker A and Walker B, are essential for phage production. The crucial role of these key residues suggests that gp1 might be a molecular motor driving phage assembly. We further identified essential residues for the function of the assembly complex. Mutations in three out of six cysteine residues abolish phage production. Similarly, two out of six conserved glycine residues are crucial for gp1 function. We hypothesise that the residues represent molecular hinges allowing domain movement for nucleotide binding and phage assembly.
Keywords: ATPase; M13; Walker motifs; assembly complex; filamentous phage; gp1; membrane protein; molecular hinge; phage assembly; secretion; zonula occludens toxin (Zot).
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


References
-
- Rakonjac J., Bennett N.J., Spagnuolo J., Gagic D., Russel M. Filamentous bacteriophage: Biology, phage display and nanotechnology applications. Curr. Issues Mol. Biol. 2011;13:51–76. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

