Second harmonic generation imaging of corneal stroma after infection by Pseudomonas aeruginosa
- PMID: 28397809
- PMCID: PMC5387403
- DOI: 10.1038/srep46116
Second harmonic generation imaging of corneal stroma after infection by Pseudomonas aeruginosa
Abstract
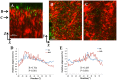
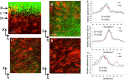
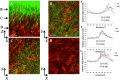
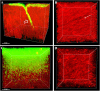
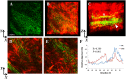
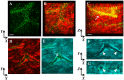
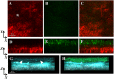
Pseudomonas aeruginosa is a pathogenic gram-negative organism that has the ability to cause blinding corneal infections following trauma and during contact lens wear. In this study, we investigated the directional movement and orientation of an invasive corneal isolate of P. aeruginosa in the corneal stroma during infection of ex vivo and in vivo rabbit corneas using multiphoton fluorescence and second harmonic generation (SHG) imaging. Ex vivo, rabbit corneas were subject to three partial thickness wounds prior to inoculation. In vivo, New Zealand white rabbits were fit with P. aeruginosa laden contact lenses in the absence of a penetrating wound. At all time points tested, infiltration of the corneal stroma by P. aeruginosa revealed a high degree of alignment between the bacteria and collagen lamellae ex vivo (p < 0.001). In vivo, P. aeruginosa traveled throughout the stroma in discrete regions or bands. Within each region, the bacteria showed good alignment with collagen lamellae (P = 0.002). Interestingly, in both the in vitro and in vivo models, P. aeruginosa did not appear to cross the corneal limbus. Taken together, our findings suggest that P. aeruginosa exploits the precise spacing of collagen lamellae in the central cornea to facilitate spread throughout the stroma.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Pachigolla G., Blomquist P. & Cavanagh H. D. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in North Texas. Eye & Contact Lens 33, 45–49 (2007). - PubMed
-
- Ormerod L. D. & Smith R. E. Contact lens-associated microbial keratitis. Arch Ophthalmol 104, 79–83 (1986). - PubMed
-
- Schein O. D. et al. Microbiology of contact lens-related keratitis. Cornea 8, 281–285 (1989). - PubMed
-
- Schein O. D., Glynn R. J., Poggio E. C., Seddon J. M. & Kenyon K. R. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N Engl J Med 321, 773–778 (1989). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

