Cardiac kinematic parameters computed from video of in situ beating heart
- PMID: 28397830
- PMCID: PMC5387404
- DOI: 10.1038/srep46143
Cardiac kinematic parameters computed from video of in situ beating heart
Abstract
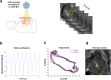
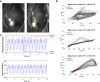
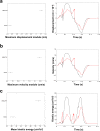
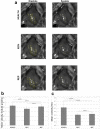
Mechanical function of the heart during open-chest cardiac surgery is exclusively monitored by echocardiographic techniques. However, little is known about local kinematics, particularly for the reperfused regions after ischemic events. We report a novel imaging modality, which extracts local and global kinematic parameters from videos of in situ beating hearts, displaying live video cardiograms of the contraction events. A custom algorithm tracked the movement of a video marker positioned ad hoc onto a selected area and analyzed, during the entire recording, the contraction trajectory, displacement, velocity, acceleration, kinetic energy and force. Moreover, global epicardial velocity and vorticity were analyzed by means of Particle Image Velocimetry tool. We validated our new technique by i) computational modeling of cardiac ischemia, ii) video recordings of ischemic/reperfused rat hearts, iii) videos of beating human hearts before and after coronary artery bypass graft, and iv) local Frank-Starling effect. In rats, we observed a decrement of kinematic parameters during acute ischemia and a significant increment in the same region after reperfusion. We detected similar behavior in operated patients. This modality adds important functional values on cardiac outcomes and supports the intervention in a contact-free and non-invasive mode. Moreover, it does not require particular operator-dependent skills.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Luciani G. B. et al.. Severe ischemic left ventricular failure: coronary operation or heart transplantation? The Annals of thoracic surgery 55, 719–723 (1993). - PubMed
-
- Wong S. C. & Leon M. B. Intracoronary stents. Current opinion in cardiology 10, 404–411 (1995). - PubMed
-
- Sipahi I., Akay M. H., Dagdelen S., Blitz A. & Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA internal medicine 174, 223–230, doi: 10.1001/jamainternmed.2013.12844 (2014). - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

