Comparison of three multiplex PCR assays for detection of respiratory viruses: Anyplex II RV16, AdvanSure RV, and Real-Q RV
- PMID: 28397965
- PMCID: PMC5836940
- DOI: 10.1002/jcla.22230
Comparison of three multiplex PCR assays for detection of respiratory viruses: Anyplex II RV16, AdvanSure RV, and Real-Q RV
Abstract
Background: Due to its great sensitivity, the nucleic acid amplification test (NAAT) is widely used for detection of respiratory viruses (RV). However, few reports have described a direct comparison between multiplex RT-PCR assays for RV. The objective of this study was to perform a direct comparison of three multiplex RT-PCR assays for the detection of respiratory viruses.
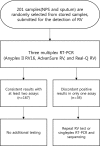
Methods: A total of 201 respiratory samples (161 nasopharyngeal swab samples and 40 sputum samples) were tested with three commercial RV assays: Seegene Anyplex II RV16 (AP), LG AdvanSure RV (AD), and Biosewoom Real-Q RV (RQ). The additional tests for the discrepant results were conducted by repeat RV assay or monoplex PCR coupled direct sequencing. Data analysis using percent agreement, kappa, and prevalence-adjusted and bias-adjusted kappa (PABAK) values was performed for comparisons among the three RV assays.
Results: Of the 201 samples, AP, AD, and RQ detected 105 (52.2%), 99 (49.3%), and 95 (47.3%) positive cases respectively. The overall agreement, kappa, and PABAK values for the three assays ranged between 97%-98%, 0.76-0.86, and 0.93-0.96 respectively. The performance of the three assays was very similar, with 94%-100% agreement for all comparisons, each virus types. The additional testing of samples showed discrepant results demonstrating that AD assay had the highest rate of concordance with original results.
Conclusions: We suggest that all multiplex assay would be suitable for the detection of for respiratory viruses in clinical setting.
Keywords: agreement; nucleic acid amplification test; respiratory virus.
© 2017 The Authors Journal of Clinical Laboratory Analysis Published by Wiley Periodicals, Inc.
Figures
References
-
- Legand A, Briand S, Shindo N, et al. Addressing the public health burden of respiratory viruses: the Battle against Respiratory Viruses (BRaVe) initiative. Future Virol. 2013;8:953‐968.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical