The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine
- PMID: 28398072
- PMCID: PMC5576088
- DOI: 10.1089/ars.2017.7083
The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine
Abstract
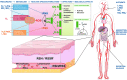
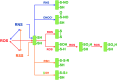
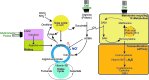
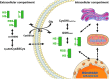
Significance: Oxidative stress is thought to account for aberrant redox homeostasis and contribute to aging and disease. However, more often than not, administration of antioxidants is ineffective, suggesting that our current understanding of the underlying regulatory processes is incomplete. Recent Advances: Similar to reactive oxygen species and reactive nitrogen species, reactive sulfur species are now emerging as important signaling molecules, targeting regulatory cysteine redox switches in proteins, affecting gene regulation, ion transport, intermediary metabolism, and mitochondrial function. To rationalize the complexity of chemical interactions of reactive species with themselves and their targets and help define their role in systemic metabolic control, we here introduce a novel integrative concept defined as the reactive species interactome (RSI). The RSI is a primeval multilevel redox regulatory system whose architecture, together with the physicochemical characteristics of its constituents, allows efficient sensing and rapid adaptation to environmental changes and various other stressors to enhance fitness and resilience at the local and whole-organism level.
Critical issues: To better characterize the RSI-related processes that determine fluxes through specific pathways and enable integration, it is necessary to disentangle the chemical biology and activity of reactive species (including precursors and reaction products), their targets, communication systems, and effects on cellular, organ, and whole-organism bioenergetics using system-level/network analyses.
Future directions: Understanding the mechanisms through which the RSI operates will enable a better appreciation of the possibilities to modulate the entire biological system; moreover, unveiling molecular signatures that characterize specific environmental challenges or other forms of stress will provide new prevention/intervention opportunities for personalized medicine. Antioxid. Redox Signal. 00, 000-000.
Keywords: hydrogen sulfide; microbiome; network medicine; nitric oxide; polysulfides; systems biology.
Conflict of interest statement
M.F. is a member of the Scientific Advisory Board of AOBiome, LLC, a company commercializing the use of ammonia-oxidizing bacteria for the treatment of inflammatory skin diseases and hypertension. None of the other authors have any conflict of interests to declare.
Figures

References
-
- Aragones J, Fraisl P, Baes M, and Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab 9: 11–22, 2009 - PubMed
-
- Bailey TS, Henthorn HA, and Pluth MD. The intersection of NO and H2S: persulfides generate NO from nitrite through polysulfide formation. Inorg Chem 55: 12618–12625, 2016 - PubMed
-
- Banne AF, Amiri A, and Pero RW. Reduced level of serum thiols in patients with a diagnosis of active disease. J Anti Aging Med 6: 327–334, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
