Emergence and evolution of an interaction between intrinsically disordered proteins
- PMID: 28398197
- PMCID: PMC5419745
- DOI: 10.7554/eLife.16059
Emergence and evolution of an interaction between intrinsically disordered proteins
Abstract
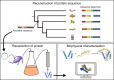
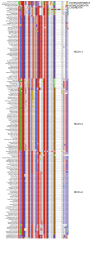
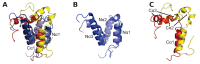
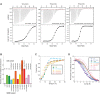
Protein-protein interactions involving intrinsically disordered proteins are important for cellular function and common in all organisms. However, it is not clear how such interactions emerge and evolve on a molecular level. We performed phylogenetic reconstruction, resurrection and biophysical characterization of two interacting disordered protein domains, CID and NCBD. CID appeared after the divergence of protostomes and deuterostomes 450-600 million years ago, while NCBD was present in the protostome/deuterostome ancestor. The most ancient CID/NCBD formed a relatively weak complex (Kd∼5 µM). At the time of the first vertebrate-specific whole genome duplication, the affinity had increased (Kd∼200 nM) and was maintained in further speciation. Experiments together with molecular modeling using NMR chemical shifts suggest that new interactions involving intrinsically disordered proteins may evolve via a low-affinity complex which is optimized by modulating direct interactions as well as dynamics, while tolerating several potentially disruptive mutations.
Keywords: Affinity; Evolution; Protein-protein interaction; biophysics; computational biology; intrinsically disordered proteins; molecular dynamics; none; phylogenetic reconstruction; structural biology; systems biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








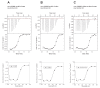
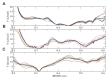
References
-
- Baftizadeh F, Cossio P, Pietrucci F, Laio A. Protein folding and Ligand-Enzyme binding from Bias-Exchange metadynamics simulations. Current Physical Chemistry. 2012;2:79–91. doi: 10.2174/1877946811202010079. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

