Genetic diversity patterns of arbuscular mycorrhizal fungi associated with the mycoheterotroph Arachnitis uniflora Phil. (Corsiaceae)
- PMID: 28398457
- PMCID: PMC5604589
- DOI: 10.1093/aob/mcx023
Genetic diversity patterns of arbuscular mycorrhizal fungi associated with the mycoheterotroph Arachnitis uniflora Phil. (Corsiaceae)
Abstract
Background and aims: Arachnitis uniflora is a mycoheterotrophic plant that exploits arbuscular mycorrhizal fungi of neighbouring plants. We tested A. uniflora 's specificity towards fungi across its large latitudinal range, as well as the role of historical events and current environmental, geographical and altitudinal variables on fungal genetic diversity.
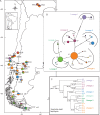
Methods: Arachnitis uniflora mycorrhizas were sampled at 25 sites. Fungal phylogenetic relationships were reconstructed, genetic diversity was calculated and the main divergent lineages were dated. Phylogeographical analysis was performed with the main fungal clade. Fungal diversity correlations with environmental factors were investigated.
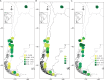
Key results: Glomeraceae fungi dominated, with a main clade that likely originated in the Upper Cretaceous and diversified in the Miocene. Two other arbuscular mycorrhizal fungal families not previously known to be targeted by A. uniflora were detected rarely and appear to be facultative associations. High genetic diversity, found in Bolivia and both northern and southern Patagonia, was correlated with temperature, rainfall and soil features.
Conclusions: Fungal genetic diversity and its distribution can be explained by the ancient evolutionary history of the target fungi and by micro-scale environmental conditions with a geographical mosaic pattern.
Keywords: Andean–Patagonian forest; Arachnitis uniflora; Arbuscular mycorrhizal fungi; genetic diversity; mycoheterotrophy; phylogeography.
© The Author 2017. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures





References
-
- Acosta MC, Premoli AC.. 2010. Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Nothofagaceae). Molecular Phylogenetics and Evolution 54: 235–242. - PubMed
-
- Acosta MC, Mathiasen P, Premoli AC.. 2014. Retracing the evolutionary history of Nothofagus in its geo-climatic context: new developments in the emerging field of phylogeology. Geobiology 12: 497–510. - PubMed
-
- Allen EB, Allen MF, Helm DJ, Trappe JM, Molina R, Rincon E.. 1995. Patterns and regulation of mycorrhizal plant and fungal diversity. Plant and Soil 170: 47–62.
-
- Anderson RC, Liberta AE.. 1992. Influence of supplemental inorganic nutrients on growth, survivorship, and mycorrhizal relationships of Schizachyrium scoparium (Poaceae) grown in fumigated and unfumigated soil. American Journal of Botany 79: 406–414.
-
- Avise JC. 2000. Phylogeography: the history and formation of species. Cambridge: Harvard University Press.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

