Deacetylation of topoisomerase I is an important physiological function of E. coli CobB
- PMID: 28398568
- PMCID: PMC5605244
- DOI: 10.1093/nar/gkx250
Deacetylation of topoisomerase I is an important physiological function of E. coli CobB
Abstract
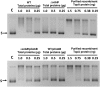
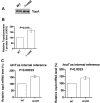
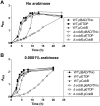
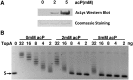
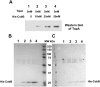
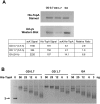
Escherichia coli topoisomerase I (TopA), a regulator of global and local DNA supercoiling, is modified by Nε-Lysine acetylation. The NAD+-dependent protein deacetylase CobB can reverse both enzymatic and non-enzymatic lysine acetylation modification in E. coli. Here, we show that the absence of CobB in a ΔcobB mutant reduces intracellular TopA catalytic activity and increases negative DNA supercoiling. TopA expression level is elevated as topA transcription responds to the increased negative supercoiling. The slow growth phenotype of the ΔcobB mutant can be partially compensated by further increase of intracellular TopA level via overexpression of recombinant TopA. The relaxation activity of purified TopA is decreased by in vitro non-enzymatic acetyl phosphate mediated lysine acetylation, and the presence of purified CobB protects TopA from inactivation by such non-enzymatic acetylation. The specific activity of TopA expressed from His-tagged fusion construct in the chromosome is inversely proportional to the degree of in vivo lysine acetylation during growth transition and growth arrest. These findings demonstrate that E. coli TopA catalytic activity can be modulated by lysine acetylation-deacetylation, and prevention of TopA inactivation from excess lysine acetylation and consequent increase in negative DNA supercoiling is an important physiological function of the CobB protein deacetylase.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Mutations reducing replication from R-loops suppress the defects of growth, chromosome segregation and DNA supercoiling in cells lacking topoisomerase I and RNase HI activity.DNA Repair (Amst). 2016 Apr;40:1-17. doi: 10.1016/j.dnarep.2016.02.001. Epub 2016 Feb 27. DNA Repair (Amst). 2016. PMID: 26947024
-
Biochemical Basis of E. coli Topoisomerase I Relaxation Activity Reduction by Nonenzymatic Lysine Acetylation.Int J Mol Sci. 2018 May 11;19(5):1439. doi: 10.3390/ijms19051439. Int J Mol Sci. 2018. PMID: 29751635 Free PMC article.
-
The E. coli sirtuin CobB shows no preference for enzymatic and nonenzymatic lysine acetylation substrate sites.Microbiologyopen. 2015 Feb;4(1):66-83. doi: 10.1002/mbo3.223. Epub 2014 Nov 22. Microbiologyopen. 2015. PMID: 25417765 Free PMC article.
-
[DNA supercoiling and topoisomerases in Escherichia coli].Rev Latinoam Microbiol. 1995 Jul-Sep;37(3):291-304. Rev Latinoam Microbiol. 1995. PMID: 8850348 Review. Spanish.
-
DNA supercoiling in vivo.Biophys Chem. 1988 Feb;29(1-2):7-15. doi: 10.1016/0301-4622(88)87020-0. Biophys Chem. 1988. PMID: 2833949 Review.
Cited by
-
Interaction between transcribing RNA polymerase and topoisomerase I prevents R-loop formation in E. coli.Nat Commun. 2022 Aug 4;13(1):4524. doi: 10.1038/s41467-022-32106-5. Nat Commun. 2022. PMID: 35927234 Free PMC article.
-
Chromosome Segregation Proteins as Coordinators of Cell Cycle in Response to Environmental Conditions.Front Microbiol. 2020 Apr 15;11:588. doi: 10.3389/fmicb.2020.00588. eCollection 2020. Front Microbiol. 2020. PMID: 32351468 Free PMC article. Review.
-
Insights into the Lysine Acetylome of the Haloarchaeon Haloferax volcanii during Oxidative Stress by Quantitative SILAC-Based Proteomics.Antioxidants (Basel). 2023 Jun 1;12(6):1203. doi: 10.3390/antiox12061203. Antioxidants (Basel). 2023. PMID: 37371933 Free PMC article.
-
Influence of Glucose Availability and CRP Acetylation on the Genome-Wide Transcriptional Response of Escherichia coli: Assessment by an Optimized Factorial Microarray Analysis.Front Microbiol. 2018 May 23;9:941. doi: 10.3389/fmicb.2018.00941. eCollection 2018. Front Microbiol. 2018. PMID: 29875739 Free PMC article.
-
The activity of CobB1 protein deacetylase contributes to nucleoid compaction in Streptomyces venezuelae spores by increasing HupS affinity for DNA.Nucleic Acids Res. 2024 Jul 8;52(12):7112-7128. doi: 10.1093/nar/gkae418. Nucleic Acids Res. 2024. PMID: 38783097 Free PMC article.
References
-
- Cain J.A., Solis N., Cordwell S.J.. Beyond gene expression: The impact of protein post-translational modifications in bacteria. J. Proteomics. 2014; 97:265–286. - PubMed
-
- Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., Olsen J.V., Mann M.. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009; 325:834–840. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

