Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants
- PMID: 28398697
- PMCID: PMC6478245
- DOI: 10.1002/14651858.CD011190.pub2
Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants
Abstract
Background: The use of supplemental oxygen in the care of extremely preterm infants has been common practice since the 1940s. Despite this, there is little agreement regarding which oxygen saturation (SpO₂) ranges to target to maximise short- or long-term growth and development, while minimising harms. There are two opposing concerns. Lower oxygen levels (targeting SpO₂ at 90% or less) may impair neurodevelopment or result in death. Higher oxygen levels (targeting SpO₂ greater than 90%) may increase severe retinopathy of prematurity or chronic lung disease.The use of pulse oximetry to non-invasively assess neonatal SpO₂ levels has been widespread since the 1990s. Until recently there were no randomised controlled trials (RCTs) that had assessed whether it is better to target higher or lower oxygen saturation levels in extremely preterm infants, from birth or soon thereafter. As a result, there is significant international practice variation and uncertainty remains as to the most appropriate range to target oxygen saturation levels in preterm and low birth weight infants.
Objectives: 1. What are the effects of targeting lower versus higher oxygen saturation ranges on death or major neonatal and infant morbidities, or both, in extremely preterm infants?2. Do these effects differ in different types of infants, including those born at a very early gestational age, or in those who are outborn, without antenatal corticosteroid coverage, of male sex, small for gestational age or of multiple birth, or by mode of delivery?
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4), MEDLINE via PubMed (1966 to 11 April 2016), Embase (1980 to 11 April 2016) and CINAHL (1982 to 11 April 2016). We also searched clinical trials databases, conference proceedings and the reference lists of retrieved articles for randomised controlled trials.
Selection criteria: Randomised controlled trials that enrolled babies born at less than 28 weeks' gestation, at birth or soon thereafter, and targeted SpO₂ ranges of either 90% or below or above 90% via pulse oximetry, with the intention of maintaining such targets for at least the first two weeks of life.
Data collection and analysis: We used the standard methods of Cochrane Neonatal to extract data from the published reports of the included studies. We sought some additional aggregate data from the original investigators in order to align the definitions of two key outcomes. We conducted the meta-analyses with Review Manager 5 software, using the Mantel-Haenszel method for estimates of typical risk ratio (RR) and risk difference (RD) and a fixed-effect model. We assessed the included studies using the Cochrane 'Risk of bias' and GRADE criteria in order to establish the quality of the evidence. We investigated heterogeneity of effects via pre-specified subgroup and sensitivity analyses.
Main results: Five trials, which together enrolled 4965 infants, were eligible for inclusion. The investigators of these five trials had prospectively planned to combine their data as part of the NeOProM (Neonatal Oxygen Prospective Meta-analysis) Collaboration. We graded the quality of evidence as high for the key outcomes of death, major disability, the composite of death or major disability, and necrotising enterocolitis; and as moderate for blindness and retinopathy of prematurity requiring treatment.When an aligned definition of major disability was used, there was no significant difference in the composite primary outcome of death or major disability in extremely preterm infants when targeting a lower (SpO₂ 85% to 89%) versus a higher (SpO₂ 91% to 95%) oxygen saturation range (typical RR 1.04, 95% confidence interval (CI) 0.98 to 1.10; typical RD 0.02, 95% CI -0.01 to 0.05; 5 trials, 4754 infants) (high-quality evidence). Compared with a higher target range, a lower target range significantly increased the incidence of death at 18 to 24 months corrected age (typical RR 1.16, 95% CI 1.03 to 1.31; typical RD 0.03, 95% CI 0.01 to 0.05; 5 trials, 4873 infants) (high-quality evidence) and necrotising enterocolitis (typical RR 1.24, 95% 1.05 to 1.47; typical RD 0.02, 95% CI 0.01 to 0.04; 5 trials, 4929 infants; I² = 0%) (high-quality evidence). Targeting the lower range significantly decreased the incidence of retinopathy of prematurity requiring treatment (typical RR 0.72, 95% CI 0.61 to 0.85; typical RD -0.04, 95% CI -0.06 to -0.02; 5 trials, 4089 infants; I² = 69%) (moderate-quality evidence). There were no significant differences between the two treatment groups for major disability including blindness, severe hearing loss, cerebral palsy, or other important neonatal morbidities.A subgroup analysis of major outcomes by type of oximeter calibration software (original versus revised) found a significant difference in the treatment effect between the two software types for death (interaction P = 0.03), with a significantly larger treatment effect seen for those infants using the revised algorithm (typical RR 1.38, 95% CI 1.13 to 1.68; typical RD 0.06, 95% CI 0.01 to 0.10; 3 trials, 1716 infants). There were no other important differences in treatment effect shown by the subgroup analyses using the currently available data.
Authors' conclusions: In extremely preterm infants, targeting lower (85% to 89%) SpO₂ compared to higher (91% to 95%) SpO₂ had no significant effect on the composite outcome of death or major disability or on major disability alone, including blindness, but increased the average risk of mortality by 28 per 1000 infants treated. The trade-offs between the benefits and harms of the different oxygen saturation target ranges may need to be assessed within local settings (e.g. alarm limit settings, staffing, baseline outcome risks) when deciding on oxygen saturation targeting policies.
Conflict of interest statement
Six members of the authorship team were investigators in the included studies and the NeOProM Collaboration. One member was included for his expertise in the field but had no affiliation with the included studies.
Lisa Askie is a member of the BOOST II Australia writing committee and the NeOProM Collaboration. Brian Darlow is a member of the BOOST‐NZ trial management committee, the BOOST II Australia trial management committee, and the NeOProM Collaboration. Peter Davis is a member of the BOOST‐II Australia trial management committee and the NeOProM Collaboration. Neil Finer is a member of the SUPPORT trial management committee and the NeOProM Collaboration. Ben Stenson is a member of the BOOST‐II UK steering committee and the NeOProM Collaboration. Maximo Vento has no conflicts of interest to declare. Robin Whyte is a member of the COT trial management committee and the NeOProM Collaboration.
Figures
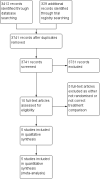




































Update of
References
References to studies included in this review
BOOST‐II Australia 2016 {published data only}
-
- Stenson BJ, Tarnow‐Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group. Oxygen saturation and outcomes in preterm infants. New England Journal of Medicine 2013;368(22):2094‐104. [DOI: 10.1056/NEJMoa1302298; PUBMED: 23642047 ] - DOI - PubMed
-
- Tarnow‐Mordi W, Stenson B, Kirby A, Juszczak E, Donoghoe M, Deshpande S, et al. BOOST‐II Australia and United Kingdom Collaborative Groups. Outcomes of two trials of oxygen‐saturation targets in preterm infants. New England Journal of Medicine 2016;374(8):749‐60. [DOI: 10.1056/NEJMoa1514212; PUBMED: 26863265 ] - DOI - PubMed
BOOST‐II UK 2016 {published data only}
-
- BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group, Stenson BJ, Tarnow‐Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. Oxygen saturation and outcomes in preterm infants. New England Journal of Medicine 2013;368(22):2094‐104. - PubMed
-
- BOOST‐II Australia and United Kingdom Collaborative Groups, Tarnow‐Mordi W, Stenson B, Kirby A, Juszczak E, Donoghoe M, Deshpande S, et al. Outcomes of two trials of oxygen‐saturation targets in preterm infants. New England Journal of Medicine 2016;374(8):749‐60. - PubMed
BOOST NZ 2014 {published and unpublished data}
-
- BOOST II United Kingdom Collaborative Group, BOOST II Australia Collaborative Group, BOOST II New Zealand Collaborative Group, Stenson BJ, Tarnow‐Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. Oxygen saturation and outcomes in preterm infants. New England Journal of Medicine 2013;368(22):2094‐104. - PubMed
-
- Darlow BA, Marschner SL, Donoghoe M, Battin MR, Broadbent RS, Elder MJ, et al. Benefits Of Oxygen Saturation Targeting‐New Zealand (BOOST‐NZ) Collaborative Group. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. Journal of Pediatrics 2014;165(1):30‐5. - PubMed
Schmidt 2013 {published and unpublished data}
-
- Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Canadian Oxygen Trial (COT) Group. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA 2013;309(20):2111‐20. [DOI: 10.1001/jama.2013.5555; NCT00637169] - DOI - PubMed
Vaucher 2012 {published and unpublished data}
-
- Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. New England Journal of Medicine 2010;362(21):1959‐69. [DOI: 10.1056/NEJMoa0911781; NCT00233324] - DOI - PMC - PubMed
-
- Vaucher YE, Peralta‐Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. New England Journal of Medicine 2012;367(26):2495‐504. [DOI: 10.1056/NEJMoa1208506; NCT00233324] - DOI - PMC - PubMed
References to studies excluded from this review
Arora 2013 {published data only}
-
- Arora V, Cayabyab R, Durand M, Ramanathan R. Graded oxygen saturation targets for premature infants in relation to outcomes. Journal of Investigative Medicine 2013;61(1):205.
Bard 1996 {published data only}
NCT00845624 {unpublished data only}
-
- NCT00845624. Time outside target oxygen saturation range in preterm infants and long term outcomes and preterm infants [Duration of time outside, below, and above the targeted oxygen saturation range in preterm infants]. https://clinicaltrials.gov/show/NCT00845624 (first received 16 February 2009).
NCT01590316 {unpublished data only}
-
- NCT01590316. SafeBoosC ‐ a phase II trial [SafeBoosC ‐ Safeguarding the brain of our smallest children ‐ an investigator‐initiated randomised, blinded, multinational, phase II feasibility clinical trial on near‐infrared spectroscopy monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants]. https://clinicaltrials.gov/show/NCT01590316 (first received 1 May 2012).
Additional references
AAP 1988
-
- American Academy of Pediatrics. Committee on Fetus and Newborn, ACOG Committee on Obstetrics: Maternal and Fetal Medicine. Guidelines for Perinatal Care. 2nd Edition. Elk Grove Village, Ill: American Academy of Pediatrics, 1988.
AAP 2002
-
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 5th Edition. American Academy of Pediatrics, 2002.
ACTRN12605000055606
-
- ACTRN12605000055606. BOOST II: Benefits Of Oxygen Saturation Targeting Study [Which oxygen saturation level should we use for very premature infants? A randomised controlled trial to investigate the effect of two slightly different oxygen levels on the health of very premature infants]. http://www.anzctr.org.au/ACTRN12605000055606.aspx (first received 14 July 2005).
ACTRN12605000253606
-
- ACTRN12605000253606. BOOST NZ [A randomised phase III study to evaluate whether a lower versus a higher oxygen saturation target in infants of <28 weeks gestation is associated with a reduction in death or disability at 2 years of age]. http://www.anzctr.org.au/ACTRN12605000253606.aspx (first received 24 August 2005).
Anderson 2003
Anderson 2004
Askie 2001a
Askie 2001b
Askie 2003
Askie 2009
Askie 2011
Avery 1960
-
- Avery ME. Recent increase in mortality from hyaline membrane disease. Journal of Pediatrics 1960;57:553‐9. [PUBMED: 13685272] - PubMed
Bolton 1974
-
- Bolton DP, Cross KW. Further observations on cost of preventing retrolental fibroplasia. Lancet 1974;1(7855):445‐8. [PUBMED: 4131442] - PubMed
Campbell 1951
-
- Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Medical Journal of Australia 1951;2(2):48‐50. [PUBMED: 14874698] - PubMed
Castillo 2008
-
- Castillo A, Sola A, Baquero H, Neira F, Alvis R, Deulofeut R, et al. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: is 85% to 93% an acceptable range?. Pediatrics 2008;121(5):882‐9. [DOI: 10.1542/peds.2007-0117; PUBMED: 18450890] - DOI - PubMed
Centre for Epi 2012
-
- Centre for Epidemiology and Evidence. New South Wales Mothers and Babies Report 2010. Sydney: NSW Ministry of Health 2012.
Chow 2003
-
- Chow LC, Wright KW, Sola A, CSMC Oxygen Administration Study Group. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants?. Pediatrics 2003;111(2):339‐45. [PUBMED: 12563061] - PubMed
Chow 2013
-
- Chow SW. The Report of the Australian and New Zealand Neonatal Network, 2010. Sydney: ANZNN, 2013.
Cross 1973
-
- Cross KW. Cost of preventing retrolental fibroplasia?. Lancet 1973;2(7835):954–6. [PUBMED: 4126572] - PubMed
Cummings 2016
Doyle 2010
Duc 1992
-
- Duc G, Sinclair JC. Oxygen administration. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn. New York: Oxford University Press, 1992:178‐99.
Fang 2016
Flynn 1992
-
- Flynn JT, Bancalari E, Snyder ES, Goldberg RN, Feuer W, Cassady J, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. New England Journal of Medicine 1992;326(16):1050‐4. [DOI: 10.1056/NEJM199204163261603; PUBMED: 1549150] - DOI - PubMed
Gao 2010
GRADEpro GDT [Computer program]
-
- Grade Working Group, McMaster University. GRADEpro [www.gradepro.org]. Version Version 14 September 2014. Hamilton (ON): Grade Working Group, McMaster University, 2014.
Hagadorn 2006
Hellstrom 2013
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ISRCTN00842661
-
- ISRCTN00842661. BOOST‐II UK [Which oxygen saturation level should we use for very premature infants? A randomised controlled trial]. http://isrctn.org/ISRCTN00842661 (first received 7 July 2006). [DOI 10.1186/ISRCTN00842661]
ISRCTN62491227
-
- ISRCTN62491227. COT [Efficacy and safety of targeting lower arterial oxygen saturations to reduce oxygen toxicity and oxidative stress in very preterm infants: the Canadian Oxygen Trial]. http://isrctn.org/ISRCTN62491227 (first received 22 August 2006). [DOI 10.1186/ISRCTN62491227]
Jobe 2001
Kapadia 2013
Kinsey 1956
-
- Kinsey VE. Retrolental fibroplasia; cooperative study of retrolental fibroplasia and the use of oxygen. A.M.A. Archives of Ophthalmology 1956;56(4):481‐543. [PUBMED: 13361620] - PubMed
Kinsey 1977
-
- Kinsey VE, Arnold HJ, Kalina RE, Stern L, Stahlman M, Odell G, et al. PaO₂ levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics 1977;60(5):655‐68. [PUBMED: 578921] - PubMed
Lanman 1954
-
- Lanman JT, Guy LP, Dancis J. Retrolental fibroplasia and oxygen therapy. Journal of the American Medical Association 1954;155(3):223‐6. [PUBMED: 13151906] - PubMed
Lim 2014
Lloyd 2003
Maltepe 2009
Manja 2015
Manja 2017
McDonald 1963
McDonald 1964
-
- McDonald AD. Oxygen treatment of premature babies and cerebral palsy. Developmental Medicine and Child Neurology 1964;6:313‐4. [PUBMED: 14155197] - PubMed
McIntosh 2001
NCT00233324
-
- NCT00233324. Surfactant Positive Airway Pressure and Pulse Oximetry Trial [Surfactant Positive Airway Pressure and Pulse Oximetry Trial (SUPPORT) in Extremely Low Birth Weight Infants]. clinicaltrials.gov/show/NCT00233324 (first received 03 October 2005).
Newburger 1984
Patz 1954
-
- Patz A. Oxygen studies in retrolental fibroplasia. IV. Clinical and experimental observations. American Journal of Ophthalmology 1954;38(3):291‐308. [PUBMED: 13188932] - PubMed
Poets 1998
-
- Poets CF. When do infants need additional inspired oxygen? A review of the current literature. Pediatric Pulmonology 1998;26(6):424‐8. [PUBMED: 9888217] - PubMed
Raju 1999
-
- Raju TN. The Nobel chronicles. 1949: Walter Rudolf Hess (1881‐1973); and Antônio Egas Moniz (1874‐1955). Lancet 1999;353(9160):1281. [PUBMED: 10217124] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saigal 2000
Saugstad 2001
-
- Saugstad OD. Update on oxygen radical disease in neonatology. Current Opinion in Obstetrics & Gynecology 2001;13(2):147‐53. [PUBMED: 11315869] - PubMed
Saugstad 2014
-
- Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta‐analysis and systematic review of the oxygen saturation target studies. Neonatology 2014;105(1):55‐63. [PUBMED: 24247112] - PubMed
Schmidt 2014
-
- Schmidt B, Whyte RK, Roberts RS. Trade‐off between lower or higher oxygen saturations for preterm infants: the first Benefits Of oxygen Saturation Targeting (BOOST) II trial reports its primary outcome. Journal of Pediatrics 2014;165(1):6‐8. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available from https://gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Silverman 1980
-
- Silverman WA. Retrolental Fibroplasia: a Modern Parable. Grune & Stratton, 1980.
Silverman 2004
-
- Silverman WA. A cautionary tale about supplemental oxygen: the albatross of neonatal medicine. Pediatrics 2004;113(2):394‐6. [PUBMED: 14754955] - PubMed
Skinner 1999
Stenson 2011
-
- Stenson B, Brocklehurst P, Tarnow‐Mordi W, UK BOOST II trial, Australian BOOST II trial, New Zealand BOOST II trial. Increased 36‐week survival with high oxygen saturation target in extremely preterm infants. New England Journal of Medicine 2011;364(17):1680‐2. [DOI: 10.1056/NEJMc1101319; PUBMED: 21524227 ] - DOI - PubMed
Stenson 2016
STOP ROP 2000
-
- Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP‐ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics 2000;105(2):295‐310. [PUBMED: 10654946] - PubMed
Subhedar 2000
Sun 2002
-
- Sun SC. Relation of target SpO₂ levels and clinical outcome in ELBW infants on supplemental oxygen. Pediatric Research 2002;51:350a.
Sutton 1999
-
- Sutton L, Bajuk B. Population‐based study of infants born at less than 28 weeks' gestation in New South Wales, Australia, in 1992‐3. New South Wales Neonatal Intensive Care Unit Study Group. Paediatric and Perinatal Epidemiology 1999;13(3):288‐301. [PUBMED: 10440049] - PubMed
Terry 1942
-
- Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens: 1. Preliminary report. American Journal of Ophthalmology 1942;25:203‐5. [PUBMED: 16693360] - PubMed
Tin 2001
Tin 2007
Usher 1961
-
- Usher RH. Clinical investigation of the respiratory distress syndrome of prematurity. Interim report. New York State Journal of Medicine 1961;61:1677‐96. [PUBMED: 13779372] - PubMed
Vento 2009
-
- Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009;124(3):e439‐49. [PUBMED: 19661049] - PubMed
Vento 2013
-
- Vento M, Teramo K. Evaluating the fetus at risk for cardio‐pulmonary compromise – what are the effects of hypoxia/hyperoxia ‐ is there a role for in‐utero resuscitation. Seminars in Fetal and Neonatal Medicine 2013;18(6):324‐9. - PubMed
Walsh 2009
-
- Walsh BK, Brooks TM, Grenier BM. Oxygen therapy in the neonatal care environment. Respiratory Care 2009;54(9):1193‐202. [PUBMED: 19712496] - PubMed
Warner 1998
-
- Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. American Journal of Physiology 1998;275(1 Pt 1):L110‐7. [PUBMED: 9688942] - PubMed
Williams 1998
Wilson 1942
-
- Wilson JL, Long SB, Howard PJ. Respiration of premature infants: response to variations of oxygen and to increased carbon dioxide in inspired air. American Journal of Diseases of Children 1942;63(6):1080‐5. [DOI: 10.1001/archpedi.1942.02010060064002] - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

