Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers Through Family-Based Outreach
- PMID: 28398847
- PMCID: PMC5501360
- DOI: 10.1200/JCO.2016.70.3439
Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers Through Family-Based Outreach
Abstract
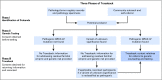
In May 2016, the Division of Cancer Prevention and the Division of Cancer Control and Population Sciences, National Cancer Institute, convened a workshop to discuss a conceptual framework for identifying and genetically testing previously diagnosed but unreferred patients with ovarian cancer and other unrecognized BRCA1 or BRCA2 mutation carriers to improve the detection of families at risk for breast or ovarian cancer. The concept, designated Traceback, was prompted by the recognition that although BRCA1 and BRCA2 mutations are frequent in women with ovarian cancer, many such women have not been tested, especially if their diagnosis predated changes in testing guidelines. The failure to identify mutation carriers among probands represents a lost opportunity to prevent cancer in unsuspecting relatives through risk-reduction intervention in mutation carriers and to provide appropriate reassurances to noncarriers. The Traceback program could provide an important opportunity to reach families from racial, ethnic, and socioeconomic groups who historically have not sought or been offered genetic counseling and testing and thereby contribute to a reduction in health disparities in women with germline BRCA mutations. To achieve an interdisciplinary perspective, the workshop assembled international experts in genetics, medical and gynecologic oncology, clinical psychology, epidemiology, genomics, cost-effectiveness modeling, pathology, bioethics, and patient advocacy to identify factors to consider when undertaking a Traceback program. This report highlights the workshop deliberations with the goal of stimulating research and providing a framework for pilot studies to assess the feasibility and ethical and logistical considerations related to the development of best practices for implementation of Traceback studies.
Figures
Comment in
-
Identification of BRCA1 and BRCA2 Mutation Carriers Through a Traceback Framework: Consent, Privacy, and Autonomy.J Clin Oncol. 2017 Jul 10;35(20):2226-2228. doi: 10.1200/JCO.2017.72.8774. Epub 2017 May 2. J Clin Oncol. 2017. PMID: 28463631 No abstract available.
References
-
- Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: Breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–594. - PubMed
-
- Eccles DM, Balmaña J, Clune J, et al. Selecting patients with ovarian cancer for germline BRCA mutation testing: Findings from guidelines and a systematic literature review. Adv Ther. 2016;33:129–150. - PubMed
-
- Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

