Cerebrovascular Remodeling and Neuroinflammation is a Late Effect of Radiation-Induced Brain Injury in Non-Human Primates
- PMID: 28398880
- PMCID: PMC5508216
- DOI: 10.1667/RR14616.1
Cerebrovascular Remodeling and Neuroinflammation is a Late Effect of Radiation-Induced Brain Injury in Non-Human Primates
Abstract
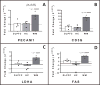
Fractionated whole-brain irradiation (fWBI) is a mainstay of treatment for patients with intracranial neoplasia; however late-delayed radiation-induced normal tissue injury remains a major adverse consequence of treatment, with deleterious effects on quality of life for affected patients. We hypothesize that cerebrovascular injury and remodeling after fWBI results in ischemic injury to dependent white matter, which contributes to the observed cognitive dysfunction. To evaluate molecular effectors of radiation-induced brain injury (RIBI), real-time quantitative polymerase chain reaction (RT-qPCR) was performed on the dorsolateral prefrontal cortex (DLPFC, Brodmann area 46), hippocampus and temporal white matter of 4 male Rhesus macaques (age 6-11 years), which had received 40 Gray (Gy) fWBI (8 fractions of 5 Gy each, twice per week), and 3 control comparators. All fWBI animals developed neurologic impairment; humane euthanasia was elected at a median of 6 months. Radiation-induced brain injury was confirmed histopathologically in all animals, characterized by white matter degeneration and necrosis, and multifocal cerebrovascular injury consisting of perivascular edema, abnormal angiogenesis and perivascular extracellular matrix deposition. Herein we demonstrate that RIBI is associated with white matter-specific up-regulation of hypoxia-associated lactate dehydrogenase A (LDHA) and that increased gene expression of fibronectin 1 (FN1), SERPINE1 and matrix metalloprotease 2 (MMP2) may contribute to cerebrovascular remodeling in late-delayed RIBI. Additionally, vascular stability and maturation associated tumor necrosis super family member 15 (TNFSF15) and vascular endothelial growth factor beta (VEGFB) mRNAs were increased within temporal white matter. We also demonstrate that radiation-induced brain injury is associated with decreases in white matter-specific expression of neurotransmitter receptors SYP, GRIN2A and GRIA4. We additionally provide evidence that macrophage/microglial mediated neuroinflammation may contribute to RIBI through increased gene expression of the macrophage chemoattractant CCL2 and macrophage/microglia associated CD68. Global patterns in cerebral gene expression varied significantly between regions examined (P < 0.0001, Friedman's test), with effects most prominent within cerebral white matter.
Figures





References
-
- Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S. Brain metastasis: opportunities in basic and translational research. Cancer Res. 2009;69(15):6015–20. - PubMed
-
- Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–9. - PubMed
-
- Hsiao KY, Yeh SA, Chang CC, Tsai PC, Wu JM, Gau JS. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77(3):722–6. - PubMed
-
- DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

