Towards better dose individualisation: metabolic phenotyping to predict cabazitaxel pharmacokinetics in men with prostate cancer
- PMID: 28399110
- PMCID: PMC5482735
- DOI: 10.1038/bjc.2017.91
Towards better dose individualisation: metabolic phenotyping to predict cabazitaxel pharmacokinetics in men with prostate cancer
Abstract
Background: Cabazitaxel is approved for treatment of castration-resistant metastatic prostate cancer. The current dosing strategy of cabazitaxel is based on body surface area (BSA). Body surface area is known as a poor predictor for total systemic exposure to drugs, since it does not take into account variability in activity of metabolising enzymes, necessary for clearance of drugs. As exposure to cabazitaxel is related to treatment response, it is essential to develop a better individualised dosing strategy.
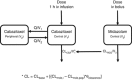
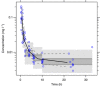
Methods: Ten patients with metastatic castration-resistant prostate cancer, who received cabazitaxel dosed on BSA as a part of routine palliative care, were enrolled in this study. Midazolam was administered as phenotyping probe for cytochrome P450 isoenzyme 3A (CYP3A). The relationship between midazolam and cabazitaxel clearance was investigated using non-linear mixed effects modelling.
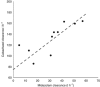
Results: The clearance of Midazolam highly correlated with cabazitaxel clearance (R=0.74). Midazolam clearance significantly (P<0.004) explained the majority (∼60%) of the inter-individual variability in cabazitaxel clearance in the studied population.
Conclusions: Metabolic phenotyping of CYP3A using midazolam is a promising strategy to individualise cabazitaxel dosing. Before clinical application, a randomised study is warranted.
Conflict of interest statement
RHJ Mathijssen receives funding by Sanofi for research projects. The remaining authors declare no conflict of interest.
Figures
Similar articles
-
Phase I study of cabazitaxel plus cisplatin in patients with advanced solid tumors: study to evaluate the impact of cytochrome P450 3A inhibitors (aprepitant, ketoconazole) or inducers (rifampin) on the pharmacokinetics of cabazitaxel.Cancer Chemother Pharmacol. 2014 Dec;74(6):1113-24. doi: 10.1007/s00280-014-2572-z. Epub 2014 Oct 12. Cancer Chemother Pharmacol. 2014. PMID: 25307552 Clinical Trial.
-
Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial.Lancet Oncol. 2017 Nov;18(11):1532-1542. doi: 10.1016/S1470-2045(17)30605-8. Epub 2017 Oct 9. Lancet Oncol. 2017. PMID: 29033099 Clinical Trial.
-
A phase I study of docetaxel with ketoconazole modulation in patients with advanced cancers.Cancer Chemother Pharmacol. 2008 Jul;62(2):243-51. doi: 10.1007/s00280-007-0598-1. Epub 2007 Oct 2. Cancer Chemother Pharmacol. 2008. PMID: 17909805 Clinical Trial.
-
Metastatic castration-resistant prostate cancer: changing landscape with cabazitaxel.Anticancer Drugs. 2014 Mar;25(3):237-43. doi: 10.1097/CAD.0000000000000045. Anticancer Drugs. 2014. PMID: 24217332 Review.
-
Cabazitaxel in metastatic castration-resistant prostate cancer.Expert Rev Anticancer Ther. 2012 Sep;12(9):1129-36. doi: 10.1586/era.12.88. Expert Rev Anticancer Ther. 2012. PMID: 23098113 Review.
Cited by
-
A Pediatric Covariate Function for CYP3A-Mediated Midazolam Clearance Can Scale Clearance of Selected CYP3A Substrates in Children.AAPS J. 2019 Jun 27;21(5):81. doi: 10.1208/s12248-019-0351-9. AAPS J. 2019. PMID: 31250333 Free PMC article.
-
Blood Concentration of Cabazitaxel in a Patient Whose General Condition Worsened with Concomitant Use of Clarithromycin.Case Rep Oncol. 2023 Jul 10;16(1):497-503. doi: 10.1159/000530547. eCollection 2023 Jan-Dec. Case Rep Oncol. 2023. PMID: 37485011 Free PMC article.
References
-
- Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48: 303–332. - PubMed
-
- Anderson BJ, Holford NH (2009) Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24: 25–36. - PubMed
-
- Bins S, Ratain MJ, Mathijssen RH (2014) Conventional dosing of anticancer agents: precisely wrong or just inaccurate? Clin Pharmacol Ther 95: 361–364. - PubMed
-
- Bracarda S, Gernone A, Gasparro D, Marchetti P, Ronzoni M, Bortolus R, Fratino L, Basso U, Mazzanti R, Messina C, Tucci M, Boccardo F, Carteni G, Pinto C, Fornarini G, Mattioli R, Procopio G, Chiuri V, Scotto T, Dondi D, Di Lorenzo G (2014) Real-world cabazitaxel safety: the Italian early-access program in metastatic castration-resistant prostate cancer. Future Oncol 10: 975–983. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

