Convergent evolution of SWS2 opsin facilitates adaptive radiation of threespine stickleback into different light environments
- PMID: 28399148
- PMCID: PMC5388470
- DOI: 10.1371/journal.pbio.2001627
Convergent evolution of SWS2 opsin facilitates adaptive radiation of threespine stickleback into different light environments
Abstract
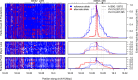
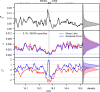
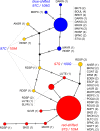
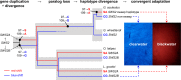
Repeated adaptation to a new environment often leads to convergent phenotypic changes whose underlying genetic mechanisms are rarely known. Here, we study adaptation of color vision in threespine stickleback during the repeated postglacial colonization of clearwater and blackwater lakes in the Haida Gwaii archipelago. We use whole genomes from 16 clearwater and 12 blackwater populations, and a selection experiment, in which stickleback were transplanted from a blackwater lake into an uninhabited clearwater pond and resampled after 19 y to test for selection on cone opsin genes. Patterns of haplotype homozygosity, genetic diversity, site frequency spectra, and allele-frequency change support a selective sweep centered on the adjacent blue- and red-light sensitive opsins SWS2 and LWS. The haplotype under selection carries seven amino acid changes in SWS2, including two changes known to cause a red-shift in light absorption, and is favored in blackwater lakes but disfavored in the clearwater habitat of the transplant population. Remarkably, the same red-shifting amino acid changes occurred after the duplication of SWS2 198 million years ago, in the ancestor of most spiny-rayed fish. Two distantly related fish species, bluefin killifish and black bream, express these old paralogs divergently in black- and clearwater habitats, while sticklebacks lost one paralog. Our study thus shows that convergent adaptation to the same environment can involve the same genetic changes on very different evolutionary time scales by reevolving lost mutations and reusing them repeatedly from standing genetic variation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Pronounced heritable variation and limited phenotypic plasticity in visual pigments and opsin expression of threespine stickleback photoreceptors.J Exp Biol. 2013 Feb 15;216(Pt 4):656-67. doi: 10.1242/jeb.078840. Epub 2012 Oct 17. J Exp Biol. 2013. PMID: 23077162
-
Rapid adaptive evolution of colour vision in the threespine stickleback radiation.Proc Biol Sci. 2016 May 11;283(1830):20160242. doi: 10.1098/rspb.2016.0242. Proc Biol Sci. 2016. PMID: 27147098 Free PMC article.
-
Experimental evidence for rapid genomic adaptation to a new niche in an adaptive radiation.Nat Ecol Evol. 2018 Jul;2(7):1128-1138. doi: 10.1038/s41559-018-0581-8. Epub 2018 Jun 25. Nat Ecol Evol. 2018. PMID: 29942074 Free PMC article.
-
Evolutionary changes of multiple visual pigment genes in the complete genome of Pacific bluefin tuna.Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11061-6. doi: 10.1073/pnas.1302051110. Epub 2013 Jun 18. Proc Natl Acad Sci U S A. 2013. PMID: 23781100 Free PMC article.
-
The genetic and molecular architecture of phenotypic diversity in sticklebacks.Philos Trans R Soc Lond B Biol Sci. 2017 Feb 5;372(1713):20150486. doi: 10.1098/rstb.2015.0486. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 27994127 Free PMC article. Review.
Cited by
-
Phenotypic divergence among threespine stickleback that differ in nuptial coloration.Ecol Evol. 2020 Feb 18;10(6):2900-2916. doi: 10.1002/ece3.6105. eCollection 2020 Mar. Ecol Evol. 2020. PMID: 32211164 Free PMC article.
-
Integrated miRNA and transcriptome profiling to explore the molecular determinism of convergent adaptation to corn in two lepidopteran pests of agriculture.BMC Genomics. 2021 Aug 9;22(1):606. doi: 10.1186/s12864-021-07905-7. BMC Genomics. 2021. PMID: 34372780 Free PMC article.
-
Opsin expression predicts male nuptial color in threespine stickleback.Ecol Evol. 2018 Jun 11;8(14):7094-7102. doi: 10.1002/ece3.4231. eCollection 2018 Jul. Ecol Evol. 2018. PMID: 30073070 Free PMC article.
-
Species coexistence through competition and rapid evolution.Proc Natl Acad Sci U S A. 2019 Feb 12;116(7):2407-2409. doi: 10.1073/pnas.1822091116. Epub 2019 Jan 28. Proc Natl Acad Sci U S A. 2019. PMID: 30692267 Free PMC article. No abstract available.
-
Multifactorial processes underlie parallel opsin loss in neotropical bats.Elife. 2018 Dec 18;7:e37412. doi: 10.7554/eLife.37412. Elife. 2018. PMID: 30560780 Free PMC article.
References
-
- Schluter D. The ecology of adaptive radiation. Oxford: Oxford University Press; 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

