Cleaning House: Selective Autophagy of Organelles
- PMID: 28399394
- PMCID: PMC5395098
- DOI: 10.1016/j.devcel.2017.02.016
Cleaning House: Selective Autophagy of Organelles
Abstract
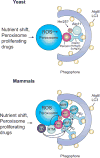
The selective clearance of organelles by autophagy is critical for the regulation of cellular homeostasis in organisms from yeast to humans. Removal of damaged organelles clears the cell of potentially toxic byproducts and enables reuse of organelle components for bioenergetics. Thus, defects in organelle clearance may be detrimental to the health of the cells, contributing to cancer, neurodegeneration, and inflammatory diseases. Organelle-specific autophagy can clear mitochondria, peroxisomes, lysosomes, ER, chloroplasts, and the nucleus. Here, we review our understanding of the mechanisms that regulate the clearance of organelles by autophagy and highlight gaps in our knowledge of these processes.
Keywords: ER-phagy; autophagy; chlorophagy; lysophagy; mitophagy; nucleophagy; pexophagy.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





References
-
- Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci. 2013;126:1905–1912. - PubMed
-
- Akinduro O, Sully K, Patel A, Robinson DJ, Chikh A, McPhail G, Braun KM, Philpott MP, Harwood CA, Byrne C, et al. Constitutive Autophagy and Nucleophagy during Epidermal Differentiation. J Invest Dermatol. 2016;136:1460–1470. - PubMed
-
- Anding AL, Baehrecke EH. Autophagy in Cell Life and Cell Death. Curr Top Dev Biol. 2015;114:67–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

