The emerging role of retromer in neuroprotection
- PMID: 28399507
- PMCID: PMC5677836
- DOI: 10.1016/j.ceb.2017.02.004
The emerging role of retromer in neuroprotection
Abstract
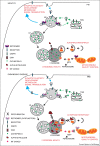
Efficient sorting and transportation of integral membrane proteins, such as ion channels, nutrient transporters, signalling receptors, cell-cell and cell-matrix adhesion molecules is essential for the function of cellular organelles and hence organism development and physiology. Retromer is a master controller of integral membrane protein sorting and transport through one of the major sorting station within eukaryotic cells, the endosomal network. Subtle de-regulation of retromer is an emerging theme in the pathoetiology of Parkinson's disease. Here we summarise recent advances in defining the neuroprotective role of retromer and how its de-regulation may contribute to Parkinson's disease by interfering with: lysosomal health and protein degradation, association with accessory proteins including the WASH complex and mitochondrial health.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Hsu V.W., Bai M., Li J. Getting active: protein sorting in endocytic recycling. Nat. Rev. Mol. Cell Biol. 2012;13:323–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

