Antacid Therapy and Disease Progression in Patients with Idiopathic Pulmonary Fibrosis Who Received Pirfenidone
- PMID: 28399537
- PMCID: PMC5452370
- DOI: 10.1159/000468546
Antacid Therapy and Disease Progression in Patients with Idiopathic Pulmonary Fibrosis Who Received Pirfenidone
Abstract
Background: Gastroesophageal reflux disease is a potential risk factor for idiopathic pulmonary fibrosis (IPF) progression; however, the impact of antacid therapy (AAT) is under debate.
Objective: To evaluate the effect of AAT on IPF progression in pirfenidone-treated patients.
Methods: This post hoc analysis included patients with IPF who received pirfenidone in 3 trials (CAPACITY [PIPF-004/PIPF-006] and ASCEND [PIPF-016]). Pulmonary function, exercise tolerance, survival, hospitalizations, and adverse events (AEs) over 52 weeks were analyzed by baseline AAT use. Disease progression was defined as a decrease in forced vital capacity (FVC) of ≥10%, a decrease in 6-min walking distance of ≥50 m, or death over 1 year.
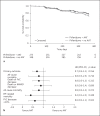
Results: Of 623 patients, 44% received AAT. No significant differences were found at 52 weeks (AAT versus non-AAT, respectively) in disease progression (24.9 vs. 30.6%; p = 0.12), all-cause mortality rate (2.9 vs. 4.0%; p = 0.47), IPF-related mortality rate (1.1 vs. 2.0%; p = 0.37), all-cause hospitalization rate (16.1 vs. 18.3%; p = 0.48), or mean change in percent FVC (-2.7 vs. -3.1%; p = 0.44). A relative, but not absolute, FVC decline of ≥10% favored AAT (15 vs. 22%; p = 0.03). Severe gastrointestinal AEs (3.7 vs. 0.9%; p = 0.015) and severe pulmonary infections (3.7 vs. 1.1%; p = 0.035) were more frequent with AAT.
Conclusions: AAT and pirfenidone had outcomes comparable to those of pirfenidone alone in patients with IPF, underscoring the need for prospective trials to elucidate the role of AAT with or without antifibrotic drugs as a treatment for IPF.
Keywords: Antacid therapy; Gastroesophageal reflux disease; Idiopathic pulmonary fibrosis; Pirfenidone; Progression-free survival.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures
Similar articles
-
Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis.Lancet Respir Med. 2016 May;4(5):381-9. doi: 10.1016/S2213-2600(16)00067-9. Epub 2016 Mar 31. Lancet Respir Med. 2016. PMID: 27050871
-
Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment.Respir Med. 2019 Jul;153:44-51. doi: 10.1016/j.rmed.2019.04.016. Epub 2019 Apr 24. Respir Med. 2019. PMID: 31153107 Clinical Trial.
-
Pirfenidone Reduces Respiratory-related Hospitalizations in Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2017 Sep 15;196(6):756-761. doi: 10.1164/rccm.201701-0091OC. Am J Respir Crit Care Med. 2017. PMID: 28471697 Free PMC article.
-
Antacid therapy in idiopathic pulmonary fibrosis: more questions than answers?Lancet Respir Med. 2017 Jul;5(7):591-598. doi: 10.1016/S2213-2600(17)30219-9. Lancet Respir Med. 2017. PMID: 28664861 Review.
-
Pirfenidone treatment of idiopathic pulmonary fibrosis.Ther Adv Respir Dis. 2012 Apr;6(2):107-14. doi: 10.1177/1753465812436663. Epub 2012 Feb 14. Ther Adv Respir Dis. 2012. PMID: 22333982 Review.
Cited by
-
Management of interstitial lung diseases: A consensus statement of the Indian Chest Society (ICS) and National College of Chest Physicians (NCCP).Lung India. 2020 Jul-Aug;37(4):359-378. doi: 10.4103/lungindia.lungindia_275_20. Lung India. 2020. PMID: 32643655 Free PMC article.
-
Management of Idiopathic Pulmonary Fibrosis.Ann Pharmacother. 2019 Dec;53(12):1238-1248. doi: 10.1177/1060028019862497. Epub 2019 Jul 7. Ann Pharmacother. 2019. PMID: 31280590 Free PMC article. Review.
-
Brazilian guidelines for the pharmacological treatment of idiopathic pulmonary fibrosis. Official document of the Brazilian Thoracic Association based on the GRADE methodology.J Bras Pneumol. 2020 Mar 2;46(2):e20190423. doi: 10.36416/1806-3756/e20190423. eCollection 2020. J Bras Pneumol. 2020. PMID: 32130337 Free PMC article.
-
Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline.Am J Respir Crit Care Med. 2022 May 1;205(9):e18-e47. doi: 10.1164/rccm.202202-0399ST. Am J Respir Crit Care Med. 2022. PMID: 35486072 Free PMC article.
-
Management of patients with fibrosing interstitial lung diseases.Am J Health Syst Pharm. 2022 Jan 24;79(3):129-139. doi: 10.1093/ajhp/zxab375. Am J Health Syst Pharm. 2022. PMID: 34608488 Free PMC article.
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR, INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. - PubMed
-
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW, ASCEND Study Group A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. - PubMed
-
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, Valeyre D, du Bois RM, CAPACITY Study Group Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. - PubMed
-
- Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, Takeuchi M, Raghu G, Kudoh S, Nukiwa T, Pirfenidone Clinical Study Group in Japan Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

