Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial
- PMID: 28399638
- PMCID: PMC6009218
- DOI: 10.2217/cns-2016-0049
Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial
Abstract
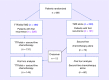
Background: This post hoc analysis of the EF-14 trial (NCT00916409) of tumor-treating fields (TTFields) plus temozolomide versus temozolomide alone in newly diagnosed glioblastoma compared the efficacy of TTFields plus chemotherapy (physician's choice) versus chemotherapy alone after first recurrence.
Methods: Patients on TTFields plus temozolomide continued TTFields plus second-line chemotherapy after first recurrence. Some patients on temozolomide alone crossed over after approval of TTFields for recurrent GBM. The primary efficacy outcome was overall survival (OS).
Results: After disease progression, 131 patients received TTFields plus chemotherapy and 73 chemotherapy alone. Thirteen patients in the original temozolomide-alone group crossed over to receive TTFields plus chemotherapy after disease progression, resulting in 144 patients receiving TTFields plus chemotherapy and 60 chemotherapy alone. Median follow-up was 12.6 months. Bevacizumab, alone or with cytotoxic chemotherapy, was the most frequent treatment. Median OS in the TTFields plus chemotherapy group was significantly longer versus chemotherapy alone (11.8 vs 9.2 months; HR: 0.70; 95% CI, 0.48-1.00; p=0.049). TTFields showed a low toxicity safety profile, as previously reported, with no grade 3/4 device-related adverse events.
Conclusion: TTFields plus chemotherapy after first disease recurrence on TTFields plus temozolomide or temozolomide alone prolonged OS in patients in the EF-14 trial.
Keywords: TTFields; bevacizumab; disease recurrence; glioblastoma; overall survival; second-line chemotherapy.
Conflict of interest statement
Figures

References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5 year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
