Comparison of atazanavir/ritonavir and darunavir/ritonavir based antiretroviral therapy for antiretroviral naïve patients
- PMID: 28399819
- PMCID: PMC5387339
- DOI: 10.1186/s12879-017-2379-8
Comparison of atazanavir/ritonavir and darunavir/ritonavir based antiretroviral therapy for antiretroviral naïve patients
Abstract
Background: Atazanavir/ritonavir and darunavir/ritonavir are common protease inhibitor-based regimens for treating patients with HIV. Studies comparing these drugs in clinical practice are lacking.
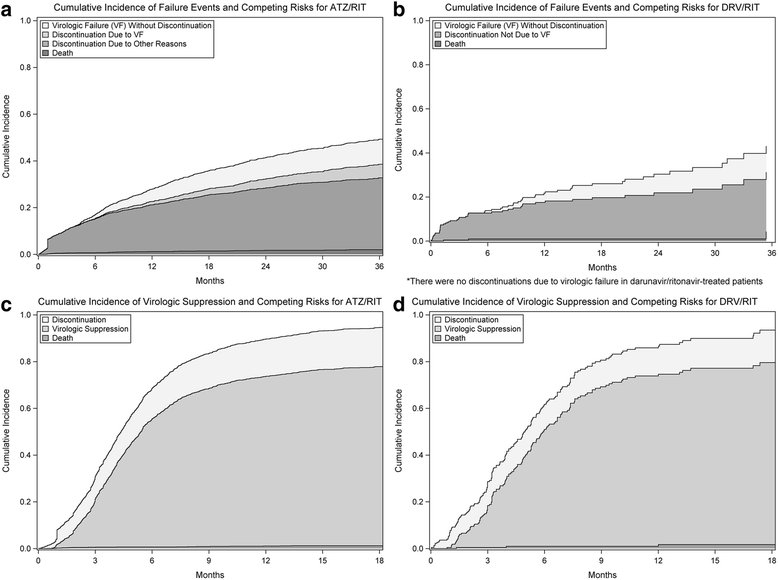
Methods: We conducted a retrospective cohort study of antiretroviral naïve participants in the Canadian Observational Cohort (CANOC) collaboration initiating atazanavir/ritonavir- or darunavir/ritonavir-based treatment. We used separate Fine and Gray competing risk regression models to compare times to regimen failure (composite of virologic failure or discontinuation for any reason). Additional endpoints included virologic failure, discontinuation due to virologic failure, discontinuation for other reasons, and virologic suppression.
Results: We studied 222 patients treated with darunavir/ritonavir and 1791 patients treated with atazanavir/ritonavir. Following multivariable adjustment, there was no difference between darunavir/ritonavir and atazanavir-ritonavir in the risk of regimen failure (adjusted hazard ratio 0.76, 95% CI 0.56 to 1.03) Darunavir/ritonavir-treated patients were at lower risk of virologic failure relative to atazanavir/ritonavir treated patients (aHR 0.50, 95% CI 0.28 to 0.91), findings driven largely by high rates of virologic failure among atazanavir/ritonavir-treated patients in the province of British Columbia. Of 108 discontinuations due to virologic failure, all occurred in patients starting atazanavir/ritonavir. There was no difference between regimens in time to discontinuation for reasons other than virologic failure (aHR 0.93; 95% CI 0.65 to 1.33) or virologic suppression (aHR 0.99, 95% CI 0.82 to 1.21).
Conclusions: The risk of regimen failure was similar between patients treated with darunavir/ritonavir and atazanavir/ritonavir. Although darunavir/ritonavir was associated with a lower risk of virologic failure relative to atazanavir/ritonavir, this difference varied substantially by Canadian province and likely reflects regional variation in prescribing practices and patient characteristics.
Keywords: Antiretroviral therapy; Atazanavir; Darunavir; HIV; Protease inhibitor.
Figures
References
-
- British HIV Association. British HIV Association guidelines for the treatment of HIV-1 positive adults with antiretroviral therapy 2015. Available at http://www.bhiva.org/documents/Guidelines/Treatment/2015/2015-treatment-.... Accessed 24 Jan 2016. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 24 Jan 2016.
-
- Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53:323–332. doi: 10.1097/QAI.0b013e3181c990bf. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical