Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study
- PMID: 28399830
- PMCID: PMC5387322
- DOI: 10.1186/s12890-017-0403-9
Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study
Abstract
Background: Electromagnetic navigation bronchoscopy (ENB) is an image-guided, minimally invasive approach that uses a flexible catheter to access pulmonary lesions.
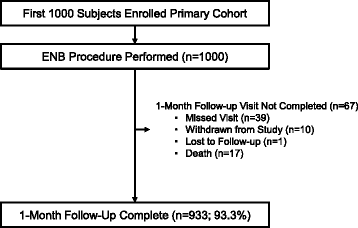
Methods: NAVIGATE is a prospective, multicenter study of the superDimension™ navigation system. A prespecified 1-month interim analysis of the first 1,000 primary cohort subjects enrolled at 29 sites in the United States and Europe is described. Enrollment and 24-month follow-up are ongoing.
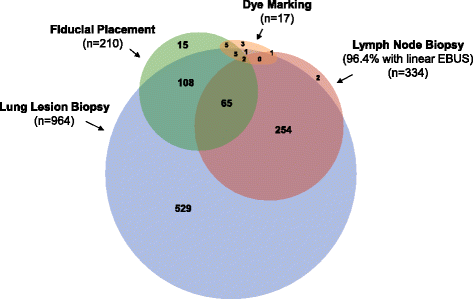
Results: ENB index procedures were conducted for lung lesion biopsy (n = 964), fiducial marker placement (n = 210), pleural dye marking (n = 17), and/or lymph node biopsy (n = 334; primarily endobronchial ultrasound-guided). Lesions were in the peripheral/middle lung thirds in 92.7%, 49.7% were <20 mm, and 48.4% had a bronchus sign. Radial EBUS was used in 54.3% (543/1,000 subjects) and general anesthesia in 79.7% (797/1,000). Among the 964 subjects (1,129 lesions) undergoing lung lesion biopsy, navigation was completed and tissue was obtained in 94.4% (910/964). Based on final pathology results, ENB-aided samples were read as malignant in 417/910 (45.8%) subjects and non-malignant in 372/910 (40.9%) subjects. An additional 121/910 (13.3%) were read as inconclusive. One-month follow-up in this interim analysis is not sufficient to calculate the true negative rate or diagnostic yield. Tissue adequacy for genetic testing was 80.0% (56 of 70 lesions sent for testing). The ENB-related pneumothorax rate was 4.9% (49/1,000) overall and 3.2% (32/1,000) CTCAE Grade ≥2 (primary endpoint). The ENB-related Grade ≥2 bronchopulmonary hemorrhage and Grade ≥4 respiratory failure rates were 1.0 and 0.6%, respectively.
Conclusions: One-month results of the first 1,000 subjects enrolled demonstrate low adverse event rates in a generalizable population across diverse practice settings. Continued enrollment and follow-up are required to calculate the true negative rate and delineate the patient, lesion, and procedural factors contributing to diagnostic yield.
Trial registration: ClinicalTrials.gov NCT02410837 . Registered 31 March 2015.
Keywords: Image-Guided Biopsy; Lung Cancer; Lung Neoplasms; Neoplasm Staging; Solitary Pulmonary Nodule.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

