Characterisation of a murine model of the late asthmatic response
- PMID: 28399855
- PMCID: PMC5387391
- DOI: 10.1186/s12931-017-0541-x
Characterisation of a murine model of the late asthmatic response
Abstract
Background: The incidence of asthma is increasing at an alarming rate. While the current available therapies are effective, there are associated side effects and they fail to adequately control symptoms in all patient subsets. In the search to understand disease pathogenesis and find effective therapies hypotheses are often tested in animal models before progressing into clinical studies. However, current dogma is that animal model data is often not predictive of clinical outcome. One possible reason for this is the end points measured such as antigen-challenge induced late asthmatic response (LAR) is often used in early clinical development, but seldom in animal model systems. As the mouse is typically selected as preferred species for pre-clinical models, we wanted to characterise and probe the validity of a murine model exhibiting an allergen induced LAR.
Methods: C57BL/6 mice were sensitised with antigen and subsequently topically challenged with the same antigen. The role of AlumTM adjuvant, glucocorticoid, long acting muscarinic receptor antagonist (LAMA), TRPA1, CD4+ and CD8+ T cells, B cells, Mast cells and IgE were determined in the LAR using genetically modified mice and a range of pharmacological tools.
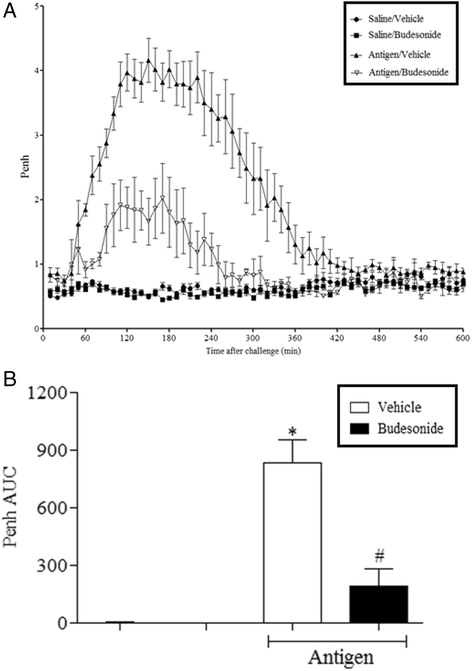
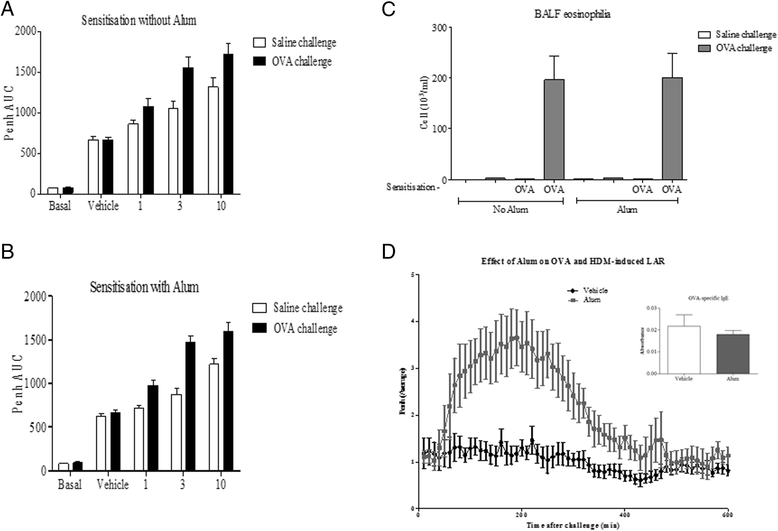
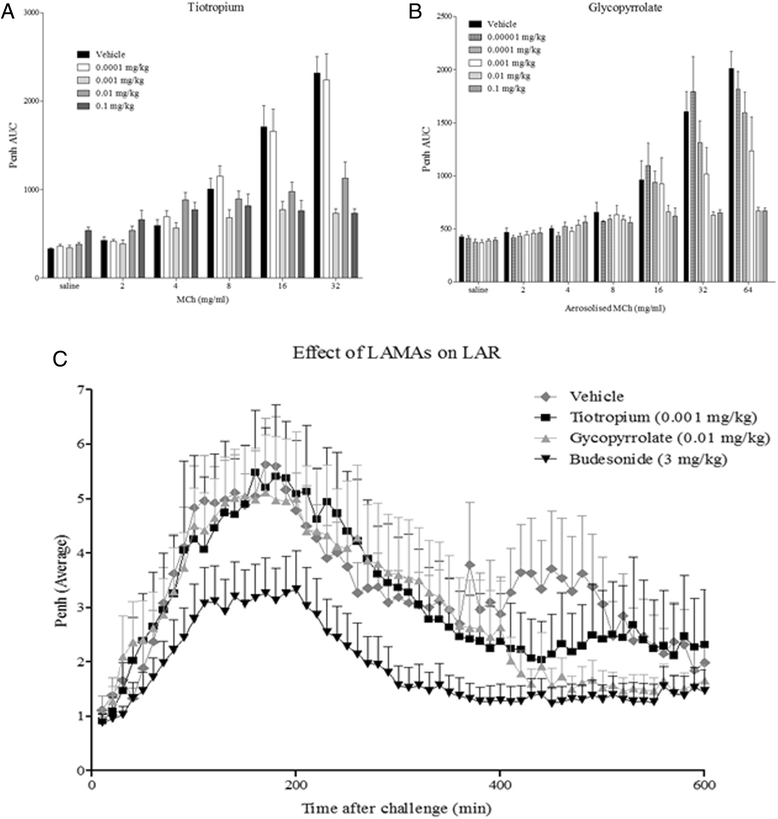
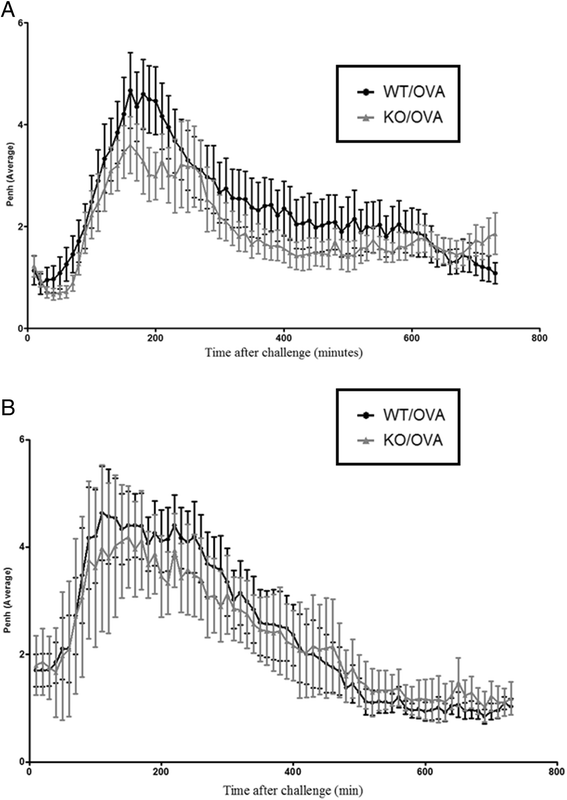
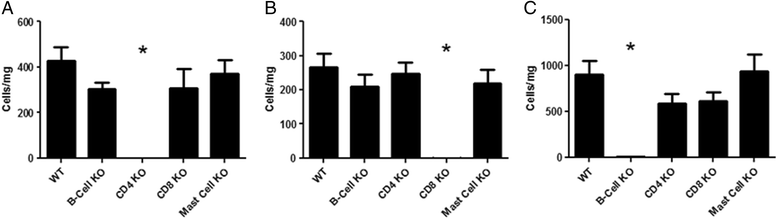
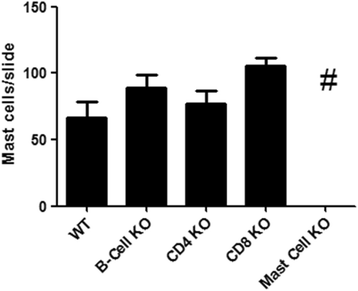
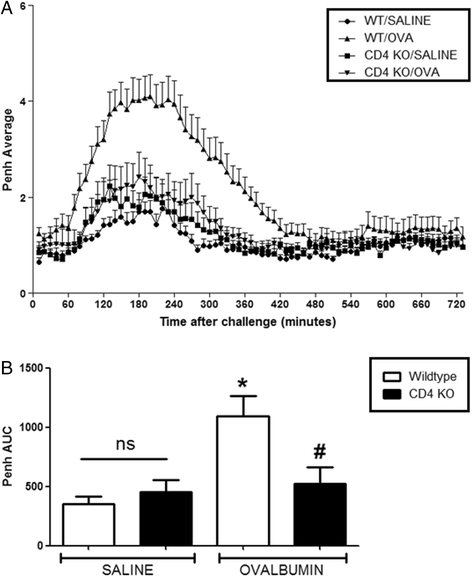
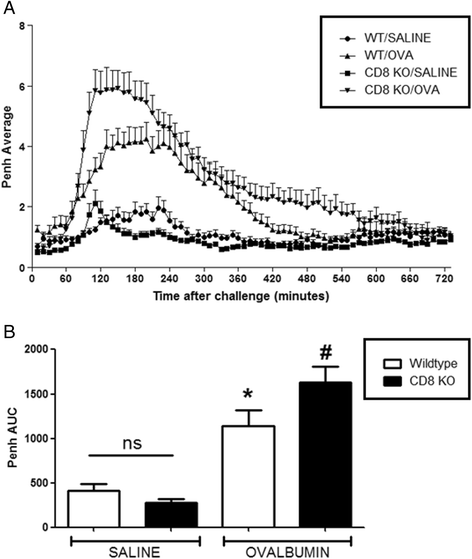
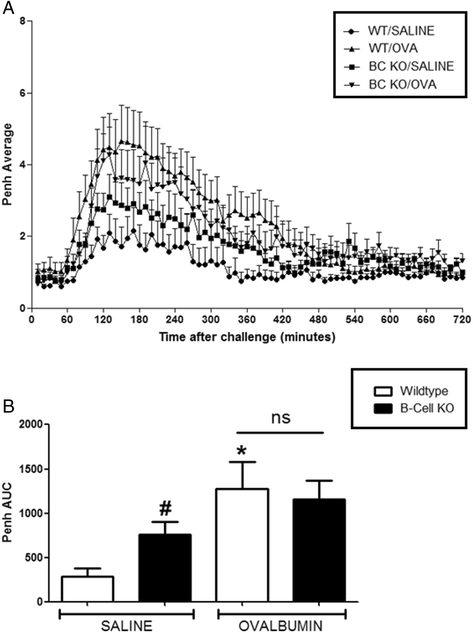
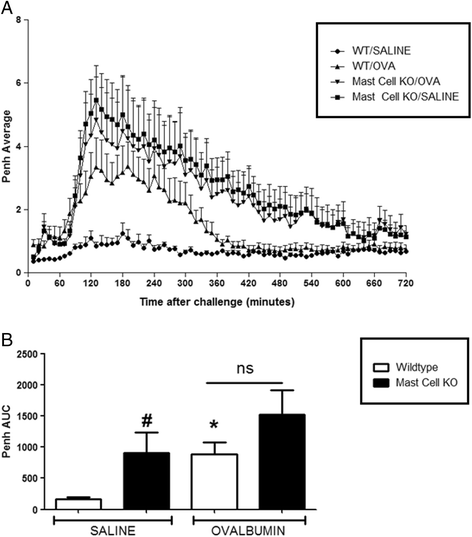
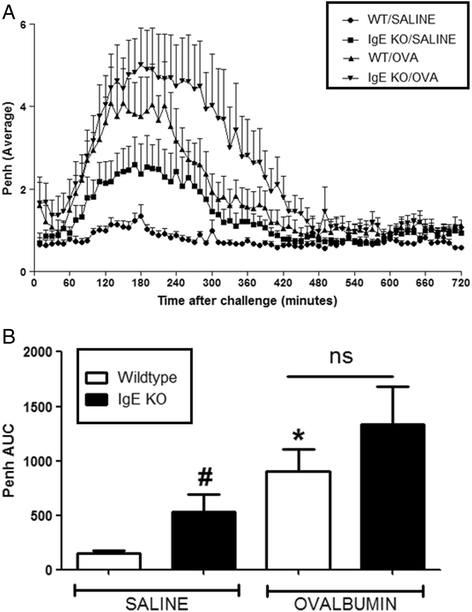
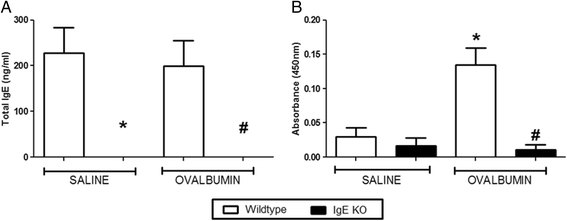
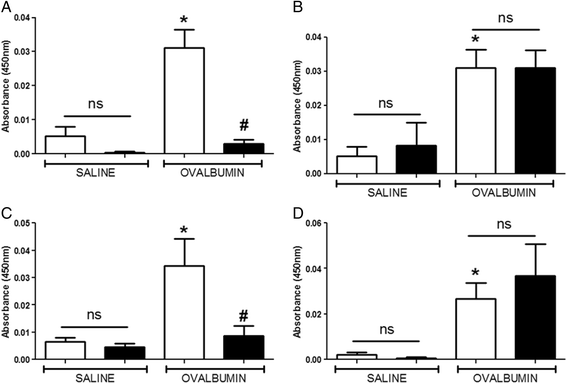
Results: Our data showed that unlike other features of asthma (e.g. cellular inflammation, elevated IgE levels and airway hyper-reactivity (AHR) the LAR required AlumTMadjuvant. Furthermore, the LAR appeared to be sensitive to glucocorticoid and required CD4+ T cells. Unlike in other species studied, the LAR was not sensitive to LAMA treatment nor required the TRPA1 ion channel, suggesting that airway sensory nerves are not involved in the LAR in this species. Furthermore, the data suggested that CD8+ T cells and the mast cell-B-cell - IgE axis appear to be protective in this murine model.
Conclusion: Together we can conclude that this model does feature steroid sensitive, CD4+ T cell dependent, allergen induced LAR. However, collectively our data questions the validity of using the murine pre-clinical model of LAR in the assessment of future asthma therapies.
Figures
Similar articles
-
CD4⁺ and CD8⁺ T cells play a central role in a HDM driven model of allergic asthma.Respir Res. 2016 Apr 25;17:45. doi: 10.1186/s12931-016-0359-y. Respir Res. 2016. PMID: 27112462 Free PMC article.
-
A role for sensory nerves in the late asthmatic response.Thorax. 2012 Jan;67(1):19-25. doi: 10.1136/thoraxjnl-2011-200365. Epub 2011 Aug 13. Thorax. 2012. PMID: 21841185
-
Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma.Respir Res. 2016 Jun 2;17(1):67. doi: 10.1186/s12931-016-0384-x. Respir Res. 2016. PMID: 27255083 Free PMC article.
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Role of immunoglobulin E and mast cells in murine models of asthma.Braz J Med Biol Res. 2003 Jul;36(7):821-7. doi: 10.1590/s0100-879x2003000700001. Epub 2003 Jun 26. Braz J Med Biol Res. 2003. PMID: 12845367 Review.
References
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2016. Available from: http://ginasthma.org/archived-reports/.
-
- World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach, 2007. Available online at http://www.who.int/gard/publications/GARD%20Book%202007.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

