Implementing isoniazid preventive therapy in a tuberculosis treatment-experienced cohort on ART
- PMID: 28399969
- PMCID: PMC5696541
- DOI: 10.5588/ijtld.16.0775
Implementing isoniazid preventive therapy in a tuberculosis treatment-experienced cohort on ART
Abstract
Setting: Urban clinical research site in Durban, South Africa.
Objective: To describe outcomes associated with the implementation of isoniazid preventive therapy (IPT) in a cohort of tuberculosis (TB) treatment-experienced human immunodeficiency virus (HIV) infected patients on antiretroviral therapy (ART).
Design: We conducted a secondary analysis of data collected between October 2009 and October 2013 from patients enrolled in a prospective cohort study conducted in Durban, South Africa.
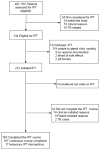
Results: Of the 402 patients enrolled in the parent study, 344 (85.6%) were eligible for IPT, 212 of whom (61.6%) initiated IPT. Of those who initiated IPT, 184 (86.8%) completed the 6-month course, while 24 (11.3%) permanently discontinued IPT, 3.8% of whom due to side effects. More women than men initiated IPT (n = 130, 61.3% vs. n = 82, 38.7%, P = 0.001). Overall median adherence to IPT was 97.6% (interquartile range 94.2-99.4). There were 22 cases of incident TB in this cohort: 13 occurred before IPT and 9 after (incidence rate ratio 0.67, 95%CI 0.29-1.58, P = 0.362).
Conclusions: IPT implementation among ART and TB treatment-experienced patients was well tolerated, with good completion rates and fewer TB cases diagnosed after IPT.
Conflict of interest statement
All authors report no conflict of interests.
Figures
References
-
- World Health Organisation. Global Tuberculosis Report 2016. Geneva, Switzerland: WHO; 2016.
-
- World Health Organisation. Factsheet No104, updated October 2016 ed. Geneva, Switzerland: WHO; Tuberculosis.
-
- World Health Organisation. Recommendation on 36 months isoniazid preventive therapy to adults and adolescents living with HIV in resource-constrained and high TB- and HIV-prevalence setting - 2015 update. Geneva, Switzerland: WHO; 2015. - PubMed
-
- South African Department of Health. National Consolidated Guidelines for the Prevention of Mother-To-Child Transmission Of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: DOH; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical