Continuous blockade of CXCR4 results in dramatic mobilization and expansion of hematopoietic stem and progenitor cells
- PMID: 28400375
- PMCID: PMC5445573
- DOI: 10.1182/blood-2016-10-746909
Continuous blockade of CXCR4 results in dramatic mobilization and expansion of hematopoietic stem and progenitor cells
Abstract
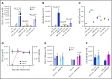
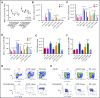
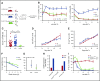
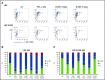
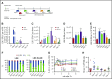
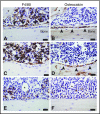
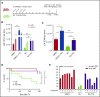
Interaction between the chemokine receptor CXCR4 and its chief ligand CXCL12 plays a critical role in the retention and migration of hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM) microenvironment. In this study, qualitative and quantitative effects of long-term pharmacologic inhibition of the CXCR4/CXCL12 axis on the HSPC compartment were investigated by using 3 structurally unrelated small molecule CXCR4 antagonists. A >10-fold increase in mobilization efficiency was achieved by administering the antagonists as a subcutaneous continuous infusion for 2 weeks compared to a single bolus injection. A concurrent increase in self-renewing proliferation leading to a twofold to fourfold expansion of the HSPC pool in the BM was observed. The expanded BM showed a distinct repopulating advantage when tested in serial competitive transplantation experiments. Furthermore, major changes within the HSPC niche associated with previously described HSPC expansion strategies were not detected in bones treated with a CXCR4 antagonist infusion. Our data suggest that prolonged but reversible pharmacologic blockade of the CXCR4/CXCL12 axis represents an approach that releases HSPC with efficiency superior to any other known mobilization strategy and may also serve as an effective method to expand the BM HSPC pool.
© 2017 by The American Society of Hematology.
Figures
References
-
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58-62. - PubMed
-
- To LB, Levesque JP, Herbert KE. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118(17):4530-4540. - PubMed
-
- Karpova D, Bonig H. Concise review: CXCR4/CXCL12 signaling in immature hematopoiesis–lessons from pharmacological and genetic models. Stem Cells. 2015;33(8):2391-2399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

