Associations of Prostate-Specific Antigen, Prostate Carcinoma Tissue Gleason Score, and Androgen Receptor Expression with Bone Metastasis in Patients with Prostate Carcinoma
- PMID: 28400549
- PMCID: PMC5398423
- DOI: 10.12659/msm.900977
Associations of Prostate-Specific Antigen, Prostate Carcinoma Tissue Gleason Score, and Androgen Receptor Expression with Bone Metastasis in Patients with Prostate Carcinoma
Abstract
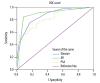
BACKGROUND Prostate carcinoma (PCa) is often not diagnosed until advanced disease with bone metastasis. Predictive factors for bone metastasis are required to improve patient outcomes. The study aimed to analyze the factors associated with bone metastases in newly diagnosed patients with PCa. MATERIAL AND METHODS This was a retrospective study of 80 patients newly diagnosed with PCa by pathological examination between January 2012 and December 2014. Bone metastases were diagnosed by positron emission computed tomography. Clinical data, serological laboratory results, and pathological examination results were collected. RESULTS Among the 80 patients, 45 (56%) had bone metastases. Age, serum alkaline phosphatase, prostate-specific antigen (PSA), erythrocyte sedimentation rate, PCa tissue Gleason score, androgen receptor (AR) expression, and Ki-67 expression were higher in patients with bone metastasis compared with those without (all P<0.05). Multivariate logistic regression showed that PSA (OR: 1.005; 95%CI: 1.001-1.010; P=0.016), Gleason score (OR: 4.095; 95%CI: 1.592-10.529; P=0.003), and AR expression (OR: 14.023; 95%CI: 3.531-55.6981; P=0.005) were independently associated with bone metastases. Cut-off values for PSA, Gleason score, and AR expression were 67.1 ng/ml (sensitivity: 55.6%; specificity: 97.1%), 7.5 (sensitivity: 75.6%; specificity: 82.9%), and 2.5 (sensitivity: 84.0%; specificity: 91.4%), respectively. CONCLUSIONS PSA, Gleason score, and AR expression in PCa tissues were independently associated with PCa bone metastases. These results could help identifying patients with PCa at high risk of bone metastases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Amato R, Stepankiw M, Gonzales P. A phase II trial of androgen deprivation therapy (ADT) plus chemotherapy as initial treatment for local failures or advanced prostate cancer. Cancer Chemother Pharmacol. 2013;71:1629–34. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer. Version 2.2014. Fort Washington: National Comprehensive Cancer Network; 2014.
-
- Graham J, Kirkbride P, Cann K, et al. Prostate cancer: Summary of updated NICE guidance. BMJ. 2014;348:f7524. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

