Kinetic CRAC uncovers a role for Nab3 in determining gene expression profiles during stress
- PMID: 28400552
- PMCID: PMC5432031
- DOI: 10.1038/s41467-017-00025-5
Kinetic CRAC uncovers a role for Nab3 in determining gene expression profiles during stress
Abstract
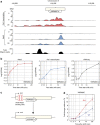
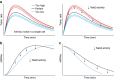
RNA-binding proteins play a key role in shaping gene expression profiles during stress, however, little is known about the dynamic nature of these interactions and how this influences the kinetics of gene expression. To address this, we developed kinetic cross-linking and analysis of cDNAs (χCRAC), an ultraviolet cross-linking method that enabled us to quantitatively measure the dynamics of protein-RNA interactions in vivo on a minute time-scale. Here, using χCRAC we measure the global RNA-binding dynamics of the yeast transcription termination factor Nab3 in response to glucose starvation. These measurements reveal rapid changes in protein-RNA interactions within 1 min following stress imposition. Changes in Nab3 binding are largely independent of alterations in transcription rate during the early stages of stress response, indicating orthogonal transcriptional control mechanisms. We also uncover a function for Nab3 in dampening expression of stress-responsive genes. χCRAC has the potential to greatly enhance our understanding of in vivo dynamics of protein-RNA interactions.Protein RNA interactions are dynamic and regulated in response to environmental changes. Here the authors describe 'kinetic CRAC', an approach that allows time resolved analyses of protein RNA interactions with minute time point resolution and apply it to gain insight into the function of the RNA-binding protein Nab3.
Conflict of interest statement
P.W. was the director and owner of UVO3 that sells equipment for water sterilization. A.L. and R.F. are employees of UVO3. P.W., A.L., and R.F. have been involved in the development of the Vari-X-linker and the filtration unit. All remaining authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

