Moderate lifelong overexpression of tuberous sclerosis complex 1 (TSC1) improves health and survival in mice
- PMID: 28400571
- PMCID: PMC5429778
- DOI: 10.1038/s41598-017-00970-7
Moderate lifelong overexpression of tuberous sclerosis complex 1 (TSC1) improves health and survival in mice
Abstract
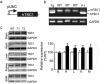
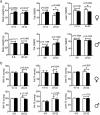
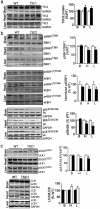
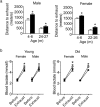
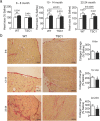
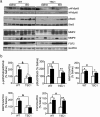
The tuberous sclerosis complex 1/2 (TSC1/2) is an endogenous regulator of the mechanistic target of rapamycin (mTOR). While mTOR has been shown to play an important role in health and aging, the role of TSC1/2 in aging has not been fully investigated. In the current study, a constitutive TSC1 transgenic (Tsc1 tg ) mouse model was generated and characterized. mTORC1 signaling was reduced in majority of the tissues, except the brain. In contrast, mTORC2 signaling was enhanced in Tsc1 tg mice. Tsc1 tg mice are more tolerant to exhaustive exercises and less susceptible to isoproterenol-induced cardiac hypertrophy at both young and advanced ages. Tsc1 tg mice have less fibrosis and inflammation in aged as well as isoproterenol-challenged heart than age-matched wild type mice. The female Tsc1 tg mice exhibit a higher fat to lean mass ratio at advanced ages than age-matched wild type mice. More importantly, the lifespan increased significantly in female Tsc1 tg mice, but not in male Tsc1 tg mice. Collectively, our data demonstrated that moderate increase of TSC1 expression can enhance overall health, particularly cardiovascular health, and improve survival in a gender-specific manner.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Constitutive adipocyte mTORC1 activation enhances mitochondrial activity and reduces visceral adiposity in mice.Biochim Biophys Acta. 2016 May;1861(5):430-8. doi: 10.1016/j.bbalip.2016.02.023. Epub 2016 Feb 27. Biochim Biophys Acta. 2016. PMID: 26923434
-
Tuberous sclerosis complex 1-mechanistic target of rapamycin complex 1 signaling determines brown-to-white adipocyte phenotypic switch.Diabetes. 2015 Feb;64(2):519-28. doi: 10.2337/db14-0427. Epub 2014 Sep 11. Diabetes. 2015. PMID: 25213336
-
TSC1 Promotes B Cell Maturation but Is Dispensable for Germinal Center Formation.PLoS One. 2015 May 22;10(5):e0127527. doi: 10.1371/journal.pone.0127527. eCollection 2015. PLoS One. 2015. PMID: 26000908 Free PMC article.
-
The tuberous sclerosis complex: balancing proliferation and survival.Biochem Soc Trans. 2011 Apr;39(2):466-71. doi: 10.1042/BST0390466. Biochem Soc Trans. 2011. PMID: 21428921 Review.
-
The Complex Roles of Mechanistic Target of Rapamycin in Adipocytes and Beyond.Trends Endocrinol Metab. 2017 May;28(5):319-339. doi: 10.1016/j.tem.2017.01.004. Epub 2017 Feb 22. Trends Endocrinol Metab. 2017. PMID: 28237819 Free PMC article. Review.
Cited by
-
The central moTOR of metabolism.Dev Cell. 2022 Mar 28;57(6):691-706. doi: 10.1016/j.devcel.2022.02.024. Epub 2022 Mar 21. Dev Cell. 2022. PMID: 35316619 Free PMC article. Review.
-
TOR-mediated regulation of metabolism in aging.Aging Cell. 2017 Dec;16(6):1219-1233. doi: 10.1111/acel.12689. Epub 2017 Oct 2. Aging Cell. 2017. PMID: 28971552 Free PMC article. Review.
-
Role of TSC1 in physiology and diseases.Mol Cell Biochem. 2021 Jun;476(6):2269-2282. doi: 10.1007/s11010-021-04088-3. Epub 2021 Feb 11. Mol Cell Biochem. 2021. PMID: 33575875 Review.
-
Targeting Autophagy in Aging and Aging-Related Cardiovascular Diseases.Trends Pharmacol Sci. 2018 Dec;39(12):1064-1076. doi: 10.1016/j.tips.2018.10.005. Epub 2018 Oct 26. Trends Pharmacol Sci. 2018. PMID: 30458935 Free PMC article. Review.
-
Mechanisms of the anti-aging and prolongevity effects of caloric restriction: evidence from studies of genetically modified animals.Aging (Albany NY). 2018 Sep 16;10(9):2243-2251. doi: 10.18632/aging.101557. Aging (Albany NY). 2018. PMID: 30222593 Free PMC article. Review.
References
-
- Dazert, E. & Hall, M. N. mTOR signaling in disease. Current opinion in cell biology, doi:10.1016/j.ceb.2011.09.003 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

