Oral and Cutaneous Lymphomas other than Mycosis Fungoides and Sézary Syndrome in a Mexican Cohort: Recategorization and Evaluation of International Geographical Disparities
- PMID: 28400635
- PMCID: PMC5363139
- DOI: 10.4103/ijd.IJD_34_17
Oral and Cutaneous Lymphomas other than Mycosis Fungoides and Sézary Syndrome in a Mexican Cohort: Recategorization and Evaluation of International Geographical Disparities
Abstract
Background: Nonmycosis fungoides/Sézary syndrome (non-MF/SS) primary cutaneous lymphomas (PCL) are currently categorized under the 2005-World Health Organization/European Organization for Research and Treatment of Cancer (WHO-EORTC) classification for PCL. These differ in behavior from secondary cutaneous lymphomas (SCL) and to lymphomas limited to the oral cavity (primary oral lymphomas [POL]) both categorized under the 2016-WHO classification for lymphoid neoplasms.
Aims: This study aims to report the first series of non-MF/SS PCL, SCL, and POL in a Mexican cohort, examine the applicability of current classification systems and compare our findings with those from foreign cohorts.
Materials and methods: Eighteen non-MF/SS PCL, four SCL, and two POL with available tissue for morphology and immunophenotypic assessment were reclassified according to the 2005-WHO/EORTC and 2016-WHO classifications.
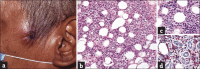
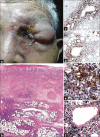
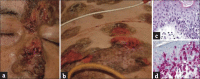
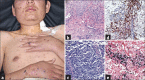
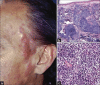
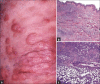
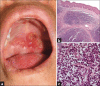
Results: Non-MF/SS PCLs were primarily of T-cell origin (61%) where CD30+ lymphoproliferative disorders predominated, followed by Epstein-Barr virus-induced lymphomas, and peripheral T-cell lymphomas, not otherwise specified. Primary cutaneous B-cell lymphomas (BCL) were primarily of follicle center cell origin followed by postgerminal lymphomas of the diffuse large BCL variety.
Conclusions: Most non-MF/SS PCL, SCL, and POL can be adequately categorized according to the 2005-WHO/EORTC and 2016-WHO classification systems, even when dealing with clinically atypical cases. The relative frequencies in our cohort hold closer similarities to Asian registries than from those of Europe/USA, supporting the concept of individual and/or racial susceptibility, and the notion of geographical variances in the rate of lymphomas. In particular, such disparity may arise from viral-induced lymphomas which might show partial geographical restriction.
Keywords: Primary cutaneous lymphomas; primary oral lymphomas; secondary cutaneous lymphomas.
Conflict of interest statement
There are no conflicts of interest. What is new? The relative frequencies of cutaneous and oral lymphomas in the first Mexican cohort hold closer similarities to Asian registries than from those of Europe and USAInternational geographical disparities in the rate of cutaneous lymphomas may arise from viral-induced lymphomas which might show partial geographical restriction.
Figures


Similar articles
-
The applicability of the new WHO-EORTC classification of primary cutaneous lymphomas to a single referral center.Am J Dermatopathol. 2008 Feb;30(1):37-44. doi: 10.1097/DAD.0b013e31815f9841. Am J Dermatopathol. 2008. PMID: 18212543
-
Clinicopathologic reassessment of non-mycosis fungoides primary cutaneous lymphomas during 17 years.Int J Dermatol. 2002 Nov;41(11):735-43. doi: 10.1046/j.1365-4362.2002.01637.x. Int J Dermatol. 2002. PMID: 12452994
-
Evaluation of the 2008 World Health Organization classification for non-mycosis fungoides, non-Sezary syndrome T/NK-cell lymphomas with primary cutaneous involvement.J Cutan Pathol. 2015 Dec;42(12):965-973. doi: 10.1111/cup.12599. Epub 2015 Sep 18. J Cutan Pathol. 2015. PMID: 26268415
-
The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants.Br J Dermatol. 2005 Nov;153(5):874-80. doi: 10.1111/j.1365-2133.2005.06905.x. Br J Dermatol. 2005. PMID: 16225594 Review.
-
Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers.J Am Acad Dermatol. 2014 Feb;70(2):205.e1-16; quiz 221-2. doi: 10.1016/j.jaad.2013.07.049. J Am Acad Dermatol. 2014. PMID: 24438969 Review.
Cited by
-
Histopathology and immunophenotyping of late onset cutaneous manifestations of COVID-19 in elderly patients: Three case reports.World J Clin Cases. 2021 Jul 16;9(20):5744-5751. doi: 10.12998/wjcc.v9.i20.5744. World J Clin Cases. 2021. PMID: 34307634 Free PMC article.
References
-
- Willemze R, Meijer CJ. EORTC classification for primary cutaneous lymphomas: A comparison with the R.E.A.L. Classification and the proposed WHO Classification. Ann Oncol. 2000;11(Suppl 1):11–5. - PubMed
-
- Willemze R, Meijer CJ. Rationale of a new classification for the group of primary cutaneous lymphomas. Semin Cutan Med Surg. 2000;19:71–7. - PubMed
-
- Willemze R, Meijer CJ. Classification of cutaneous T-cell lymphoma: From Alibert to WHO-EORTC. J Cutan Pathol. 2006;33(Suppl 1):18–26. - PubMed
-
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. - PubMed
-
- Burg G, Kempf W, Cozzio A, Feit J, Willemze R, S Jaffe E, et al. WHO/EORTC classification of cutaneous lymphomas 2005: Histological and molecular aspects. J Cutan Pathol. 2005;32:647–74. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
