Characterization of NO-Induced Nitrosative Status in Human Placenta from Pregnant Women with Gestational Diabetes Mellitus
- PMID: 28400911
- PMCID: PMC5376459
- DOI: 10.1155/2017/5629341
Characterization of NO-Induced Nitrosative Status in Human Placenta from Pregnant Women with Gestational Diabetes Mellitus
Abstract
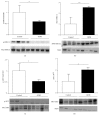
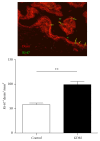
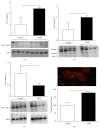
Dysregulation of NO production is implicated in pregnancy-related diseases, including gestational diabetes mellitus (GDM). The role of NO and its placental targets in GDM pregnancies has yet to be determined. S-Nitrosylation is the NO-derived posttranslational protein modification that can modulate biological functions by forming NO-derived complexes with longer half-life, termed S-nitrosothiol (SNO). Our aim was to examine the presence of endogenous S-nitrosylated proteins in cysteine residues in relation to antioxidant defense, apoptosis, and cellular signal transduction in placental tissue from control (n = 8) and GDM (n = 8) pregnancies. S-Nitrosylation was measured using the biotin-switch assay, while the expression and protein activity were assessed by immunoblotting and colorimetric methods, respectively. Results indicated that catalase and peroxiredoxin nitrosylation levels were greater in GDM placentas, and that was accompanied by reduced catalase activity. S-Nitrosylation of ERK1/2 and AKT was increased in GDM placentas, and their activities were inhibited. Activities of caspase-3 and caspase-9 were increased, with the latter also showing diminished nitrosylation levels. These findings suggest that S-nitrosylation is a little-known, but critical, mechanism by which NO directly modulates key placental proteins in women with GDM and, as a consequence, maternal and fetal anomalies during pregnancy can occur.
Figures


Similar articles
-
No evidence of attenuation of placental insulin-stimulated Akt phosphorylation and amino acid transport in maternal obesity and gestational diabetes mellitus.Am J Physiol Endocrinol Metab. 2019 Dec 1;317(6):E1037-E1049. doi: 10.1152/ajpendo.00196.2019. Epub 2019 Oct 1. Am J Physiol Endocrinol Metab. 2019. PMID: 31573844 Free PMC article.
-
BMI-Independent Effects of Gestational Diabetes on Human Placenta.J Clin Endocrinol Metab. 2018 Sep 1;103(9):3299-3309. doi: 10.1210/jc.2018-00397. J Clin Endocrinol Metab. 2018. PMID: 29931171
-
Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium.J Clin Endocrinol Metab. 2012 Oct;97(10):3613-21. doi: 10.1210/jc.2012-1212. Epub 2012 Aug 14. J Clin Endocrinol Metab. 2012. PMID: 22893718
-
Nitrosative Stress in the Nervous System: Guidelines for Designing Experimental Strategies to Study Protein S-Nitrosylation.Neurochem Res. 2016 Mar;41(3):510-4. doi: 10.1007/s11064-015-1640-z. Epub 2015 Jun 29. Neurochem Res. 2016. PMID: 26118537 Free PMC article. Review.
-
Proteomic approaches to evaluate protein S-nitrosylation in disease.Mass Spectrom Rev. 2014 Jan-Feb;33(1):7-20. doi: 10.1002/mas.21373. Epub 2013 Jun 15. Mass Spectrom Rev. 2014. PMID: 23775552 Review.
Cited by
-
Blunted Reducing Power Generation in Erythrocytes Contributes to Oxidative Stress in Prepubertal Obese Children with Insulin Resistance.Antioxidants (Basel). 2021 Feb 5;10(2):244. doi: 10.3390/antiox10020244. Antioxidants (Basel). 2021. PMID: 33562490 Free PMC article.
-
Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds.Molecules. 2021 Jul 5;26(13):4105. doi: 10.3390/molecules26134105. Molecules. 2021. PMID: 34279445 Free PMC article. Review.
-
Placental Nutrient Transport in Gestational Diabetic Pregnancies.Front Endocrinol (Lausanne). 2017 Nov 7;8:306. doi: 10.3389/fendo.2017.00306. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29163373 Free PMC article. Review.
-
Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity.Antioxidants (Basel). 2020 Aug 26;9(9):794. doi: 10.3390/antiox9090794. Antioxidants (Basel). 2020. PMID: 32859037 Free PMC article.
-
The human placenta and its role in reproductive outcomes revisited.Physiol Rev. 2025 Oct 1;105(4):2305-2376. doi: 10.1152/physrev.00039.2024. Epub 2025 Jun 11. Physiol Rev. 2025. PMID: 40497429 Review.
References
-
- Moncada S., Palmer R. M. J., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43(2):109–142. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

