Impact on Autophagy and Ultraviolet B Induced Responses of Treatment with the MTOR Inhibitors Rapamycin, Everolimus, Torin 1, and pp242 in Human Keratinocytes
- PMID: 28400912
- PMCID: PMC5376460
- DOI: 10.1155/2017/5930639
Impact on Autophagy and Ultraviolet B Induced Responses of Treatment with the MTOR Inhibitors Rapamycin, Everolimus, Torin 1, and pp242 in Human Keratinocytes
Abstract
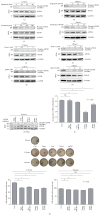
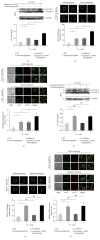
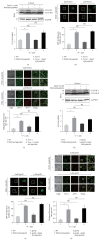
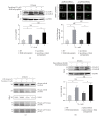
The mechanistic target of Rapamycin (MTOR) protein is a crucial signaling regulator in mammalian cells that is extensively involved in cellular biology. The function of MTOR signaling in keratinocytes remains unclear. In this study, we detected the MTOR signaling and autophagy response in the human keratinocyte cell line HaCaT and human epidermal keratinocytes treated with MTOR inhibitors. Moreover, we detected the impact of MTOR inhibitors on keratinocytes exposed to the common carcinogenic stressors ultraviolet B (UVB) and UVA radiation. As a result, keratinocytes were sensitive to the MTOR inhibitors Rapamycin, everolimus, Torin 1, and pp242, but the regulation of MTOR downstream signaling was distinct. Next, autophagy induction only was observed in HaCaT cells treated with Rapamycin. Furthermore, we found that MTOR signaling was insensitive to UVB but sensitive to UVA radiation. UVB treatment also had no impact on the inhibition of MTOR signaling by MTOR inhibitors. Finally, MTOR inhibition by Rapamycin, everolimus, or pp242 did not affect the series of biological events in keratinocytes exposed to UVB, including the downregulation of BiP and PERK, activation of Histone H2A and JNK, and cleavage of caspase-3 and PARP. Our study demonstrated that MTOR inhibition in keratinocytes cannot always induce autophagy, and the MTOR pathway does not play a central role in the UVB triggered cellular response.
Figures



Similar articles
-
Ultraviolet B radiation down-regulates ULK1 and ATG7 expression and impairs the autophagy response in human keratinocytes.J Photochem Photobiol B. 2018 Jan;178:152-164. doi: 10.1016/j.jphotobiol.2017.08.043. Epub 2017 Sep 4. J Photochem Photobiol B. 2018. PMID: 29154199
-
Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention.Cell Signal. 2016 May;28(5):460-468. doi: 10.1016/j.cellsig.2016.02.008. Epub 2016 Feb 12. Cell Signal. 2016. PMID: 26876613 Free PMC article.
-
mTOR kinase inhibitor pp242 causes mitophagy terminated by apoptotic cell death in E1A-Ras transformed cells.Oncotarget. 2015 Dec 29;6(42):44905-26. doi: 10.18632/oncotarget.6457. Oncotarget. 2015. PMID: 26636543 Free PMC article.
-
Cellular and molecular effects of the mTOR inhibitor everolimus.Clin Sci (Lond). 2015 Nov;129(10):895-914. doi: 10.1042/CS20150149. Clin Sci (Lond). 2015. PMID: 26330617 Review.
-
mTOR Inhibition Role in Cellular Mechanisms.Transplantation. 2018 Feb;102(2S Suppl 1):S3-S16. doi: 10.1097/TP.0000000000001806. Transplantation. 2018. PMID: 29369970 Review.
Cited by
-
Rapamycin Treatment Reduces Acute Myocarditis Induced by Trypanosoma cruzi Infection.J Innate Immun. 2020;12(4):321-332. doi: 10.1159/000504322. Epub 2019 Dec 4. J Innate Immun. 2020. PMID: 31801138 Free PMC article.
-
Combined mTORC1/mTORC2 inhibition blocks growth and induces catastrophic macropinocytosis in cancer cells.Proc Natl Acad Sci U S A. 2019 Dec 3;116(49):24583-24592. doi: 10.1073/pnas.1911393116. Epub 2019 Nov 15. Proc Natl Acad Sci U S A. 2019. PMID: 31732667 Free PMC article.
-
Lycopene upregulates ZO-1 and downregulates claudin-1 through autophagy inhibition in the human cutaneous squamous cell carcinoma cell line COLO-16.J Cancer. 2019 Jan 1;10(2):510-521. doi: 10.7150/jca.26578. eCollection 2019. J Cancer. 2019. PMID: 30719147 Free PMC article.
-
Regulated cell death (RCD) in cancer: key pathways and targeted therapies.Signal Transduct Target Ther. 2022 Aug 13;7(1):286. doi: 10.1038/s41392-022-01110-y. Signal Transduct Target Ther. 2022. PMID: 35963853 Free PMC article. Review.
-
Crosstalk between the mTOR and DNA Damage Response Pathways in Fission Yeast.Cells. 2021 Feb 2;10(2):305. doi: 10.3390/cells10020305. Cells. 2021. PMID: 33540829 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

