Gpr110 deficiency decelerates carcinogen-induced hepatocarcinogenesis via activation of the IL-6/STAT3 pathway
- PMID: 28401002
- PMCID: PMC5385634
Gpr110 deficiency decelerates carcinogen-induced hepatocarcinogenesis via activation of the IL-6/STAT3 pathway
Abstract
Hepatocarcinogenesis is a complex process that includes pronounced necroinflammation, unregulated hepatocyte damage, subsequent extensive fibrosis, and carcinogenesis. GPR110 was an adhesion G protein-coupled receptor. Analysis of the expression pattern of Gpr110 in mice displayed that Gpr110 was expressed highly in liver, implicating the tissue compartments where Gpr110 could execute its functions, the role of Gpr110 in the physiological and pathological state of liver remains unclear. Based on a Gpr110 knockout mouse model, we evaluated the role of Gpr110 in hepatocarcinogenesis by using a carbon tetrachloride (CCl4)-induced liver injury and fibrosis model, as well as diethylnitrosamine (DEN) plus CCl4-induced liver cancer model. In this study, we found subdued chronic liver injury, reduced compensatory proliferation, lower liver fibrosis, but enhanced inflammation occurred in Gpr110-/- mice during CCl4 challenge. In addition, Gpr110-/- mice were resistant to liver tumorigenesis induced by DEN plus CCl4 injection. Molecular mechanisms underlying these differences correlated with augmented activation of the IL-6/STAT3 pathway, which exerted hepatoprotective effects during liver damage, fibrosis, and oncogenesis in Gpr110-/- mice. Furthermore, pharmacological inhibition of the activation of the IL-6/STAT3 pathway enhanced hepatic fibrosis and promoted DEN plus CCl4-induced carcinogenesis in Gpr110-/- mice. In summary, absence of Gpr110 decelerates liver fibrosis/cirrhosis progressing into tumorigenesis, due to strengthening activation of the IL-6/STAT3 pathway, leading to a weaker liver injury and fibrosis microenvironment. It is indicated that targeting Gpr110 and activating the IL-6/STAT3 pathway may be considered to be preventive methods for some cirrhosis transition.
Keywords: CCl4; Gpr110; fibrosis; hepatocarcinogenesis; knockout mouse model.
Figures


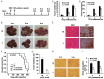

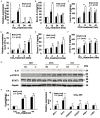
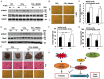
Similar articles
-
The role of GPR110 in lung cancer progression.Ann Transl Med. 2020 Jun;8(12):745. doi: 10.21037/atm-20-3146. Ann Transl Med. 2020. PMID: 32647670 Free PMC article.
-
Gankyrin drives malignant transformation of chronic liver damage-mediated fibrosis via the Rac1/JNK pathway.Cell Death Dis. 2015 May 7;6(5):e1751. doi: 10.1038/cddis.2015.120. Cell Death Dis. 2015. PMID: 25950481 Free PMC article.
-
Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis.Am J Pathol. 2011 Aug;179(2):714-24. doi: 10.1016/j.ajpath.2011.05.005. Epub 2011 Jun 2. Am J Pathol. 2011. PMID: 21684247 Free PMC article.
-
Fibrosis-associated hepatocarcinogenesis revisited: Establishing standard medium-term chemically-induced male and female models.PLoS One. 2018 Sep 13;13(9):e0203879. doi: 10.1371/journal.pone.0203879. eCollection 2018. PLoS One. 2018. PMID: 30212575 Free PMC article.
-
STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib.J Autoimmun. 2013 Oct;46:25-34. doi: 10.1016/j.jaut.2013.07.008. Epub 2013 Aug 13. J Autoimmun. 2013. PMID: 23948302
Cited by
-
Clinical Significance of G Protein-Coupled Receptor 110 (GPR110) as a Novel Prognostic Biomarker in Osteosarcoma.Med Sci Monit. 2018 Jul 27;24:5216-5224. doi: 10.12659/MSM.909555. Med Sci Monit. 2018. PMID: 30052620 Free PMC article.
-
Amelioration of non-alcoholic fatty liver disease by targeting adhesion G protein-coupled receptor F1 (Adgrf1).Elife. 2023 Aug 15;12:e85131. doi: 10.7554/eLife.85131. Elife. 2023. PMID: 37580962 Free PMC article.
-
Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine.Br J Pharmacol. 2019 May;176(10):1443-1454. doi: 10.1111/bph.14175. Epub 2018 Apr 2. Br J Pharmacol. 2019. PMID: 29473944 Free PMC article. Review.
-
The role of GPR110 in lung cancer progression.Ann Transl Med. 2020 Jun;8(12):745. doi: 10.21037/atm-20-3146. Ann Transl Med. 2020. PMID: 32647670 Free PMC article.
-
A novel role of ADGRF1 (GPR110) in promoting cellular quiescence and chemoresistance in human epidermal growth factor receptor 2-positive breast cancer.FASEB J. 2021 Jul;35(7):e21719. doi: 10.1096/fj.202100070R. FASEB J. 2021. PMID: 34110646 Free PMC article.
References
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
-
- Mu X, Espanol-Suner R, Mederacke I, Affo S, Manco R, Sempoux C, Lemaigre FP, Adili A, Yuan D, Weber A, Unger K, Heikenwalder M, Leclercq IA, Schwabe RF. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J Clin Invest. 2015;125:3891–3903. - PMC - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. - PubMed
-
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
