Integrin β1 is a critical effector in promoting metastasis and chemo-resistance of esophageal squamous cell carcinoma
- PMID: 28401009
- PMCID: PMC5385641
Integrin β1 is a critical effector in promoting metastasis and chemo-resistance of esophageal squamous cell carcinoma
Abstract
Metastasis of esophageal squamous cell carcinoma (ESCC) remains a challenge in clinical practice. In this study, we clarified that integrin β1 (ITGB1) plays critical roles in the metastasis of ESCC. By analyzing the expression of integrin β1 in ESCC specimens, we found that the expression of this integrin was higher in malignant than in normal tissues and that this increase was associated with lymph node metastasis. Moreover, in vitro functional experiments demonstrated that deletion of integrin β1 impaired the motility of ESCC cells, and we also showed that integrin β1 deletion significantly inhibited metastases formation in the lungs and lymph nodes of two murine models. Mechanistically, integrin β1 promoted cellular motility by regulating the FAK-Rac1 signaling pathway. Finally, we found that blocking integrin β1 significantly impaired the resistance of ESCC cells to cisplatin (DDP) treatment based on in vitro and in vivo experiments. Overall, our data suggest that integrin β1 promotes metastasis and confers DDP resistance to ESCC, which provides experimental evidence for targeting this protein to treat ESCC in the future.
Keywords: FAK; Integrin β1; chemo-resistance; esophageal cancer; metastasis.
Figures
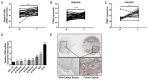
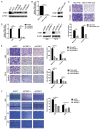


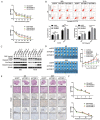
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Itou J, Tanaka S, Li W, Iida A, Sehara-Fujisawa A, Sato F, Toi M. The Sal-like 4-integrin alpha6beta1 network promotes cell migration for metastasis via activation of focal adhesion dynamics in basal-like breast cancer cells. Biochim Biophys Acta. 2017;1864:76–88. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
