Inhibition of STAT3 signaling blocks obesity-induced mammary hyperplasia in a mouse model
- PMID: 28401024
- PMCID: PMC5385655
Inhibition of STAT3 signaling blocks obesity-induced mammary hyperplasia in a mouse model
Abstract
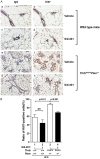
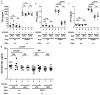
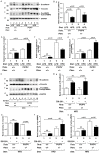
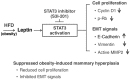
Compelling epidemiologic evidence indicates that obesity is a risk factor for human cancers, including breast. However, molecular mechanisms by which obesity could contribute to the development of breast cancer remain unclear. To understand the impact of obesity on breast cancer development, we used a mutant mouse that expresses a mutated thyroid hormone receptor β (denoted as PV) with haplodeficiency of the Pten gene (ThrbPV/PVPten+/- mice). We previously showed that adult nulliparous female ThrbPV/PVPten+/- mice developed extensive mammary hyperplasia and breast tumors. In this study, we induced obesity in ThrbPV/PVPten+/- mice by feeding them a high fat diet (HFD). We found HFD exacerbated the extent of mammary hyperplasia in ThrbPV/PVPten+/- mice. HFD elevated serum leptin levels but had no effect on the levels of serum thyroid stimulating hormone, thyroid hormones, and estrogens. Molecular analysis showed that the obesity-induced hyperplasia was mediated by the leptin/leptin receptor-JAK1-STAT3 pathway to increase key cell cycle regulators to stimulate mammary epithelial cell proliferation. Activated STAT3 signaling led to altered expression in the key regulators of epithelial-mesenchymal-transition (EMT) to augment invasiveness and migration of mammary proliferating epithelial cells. Moreover, treatment of HFD-ThrbPV/PVPten+/- mice with a STAT3 inhibitor, S3I-201, markedly reversed the obesity-induced mammary hyperplasia and reduced EMT signals to lessen cell invasiveness and migration. Our studies not only elucidated how obesity could contribute to mammary hyperplasia at the molecular level, but also, importantly, demonstrated that inhibition of the STAT3 activity could be a novel treatment strategy for obesity-induced breast cancer progression.
Keywords: JAK2-STAT3 signaling; Mammary carcinogenesis; STAT3 inhibitor; obesity; preclinical mouse model; thyroid hormone receptor β mutant.
Figures


References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Rose DP, Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther. 2009;9:1091–1101. - PubMed
-
- Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123:641–649. - PubMed
-
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. - PubMed
-
- Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF, Urrutia RP, Knudtson J, Anderson GL. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015;1:611–621. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
