SCM, the M Protein of Streptococcus canis Binds Immunoglobulin G
- PMID: 28401063
- PMCID: PMC5368172
- DOI: 10.3389/fcimb.2017.00080
SCM, the M Protein of Streptococcus canis Binds Immunoglobulin G
Abstract
The M protein of Streptococcus canis (SCM) is a virulence factor and serves as a surface-associated receptor with a particular affinity for mini-plasminogen, a cleavage product of the broad-spectrum serine protease plasmin. Here, we report that SCM has an additional high-affinity immunoglobulin G (IgG) binding activity. The ability of a particular S. canis isolate to bind to IgG significantly correlates with a scm-positive phenotype, suggesting a dominant role of SCM as an IgG receptor. Subsequent heterologous expression of SCM in non-IgG binding S. gordonii and Western Blot analysis with purified recombinant SCM proteins confirmed its IgG receptor function. As expected for a zoonotic agent, the SCM-IgG interaction is species-unspecific, with a particular affinity of SCM for IgGs derived from human, cats, dogs, horses, mice, and rabbits, but not from cows and goats. Similar to other streptococcal IgG-binding proteins, the interaction between SCM and IgG occurs via the conserved Fc domain and is, therefore, non-opsonic. Interestingly, the interaction between SCM and IgG-Fc on the bacterial surface specifically prevents opsonization by C1q, which might constitute another anti-phagocytic mechanism of SCM. Extensive binding analyses with a variety of different truncated SCM fragments defined a region of 52 amino acids located in the central part of the mature SCM protein which is important for IgG binding. This binding region is highly conserved among SCM proteins derived from different S. canis isolates but differs significantly from IgG-Fc receptors of S. pyogenes and S. dysgalactiae sub. equisimilis, respectively. In summary, we present an additional role of SCM in the pathogen-host interaction of S. canis. The detailed analysis of the SCM-IgG interaction should contribute to a better understanding of the complex roles of M proteins in streptococcal pathogenesis.
Keywords: Immunoglobulin G; M protein; Streptococcus canis; anti-phagocytic activity; zoonosis.
Figures



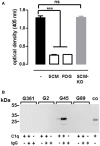
References
-
- Berggard K., Johnsson E., Morfeldt E., Persson J., Stalhammar-Carlemalm M., Lindahl G. (2001). Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol. 42, 539–551. 10.1046/j.1365-2958.2001.02664.x - DOI - PubMed
-
- Bjorck L., Kronvall G. (1984). Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J. Immunol. 133, 969–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

