History and future technical innovation in positron emission tomography
- PMID: 28401173
- PMCID: PMC5374360
- DOI: 10.1117/1.JMI.4.1.011013
History and future technical innovation in positron emission tomography
Abstract
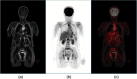
Instrumentation for positron emission tomography (PET) imaging has experienced tremendous improvements in performance over the past 60 years since it was first conceived as a medical imaging modality. Spatial resolution has improved by a factor of 10 and sensitivity by a factor of 40 from the early designs in the 1970s to the high-performance scanners of today. Multimodality configurations have emerged that combine PET with computed tomography (CT) and, more recently, with MR. Whole-body scans for clinical purposes can now be acquired in under 10 min on a state-of-the-art PET/CT. This paper will review the history of these technical developments over 40 years and summarize the important clinical research and healthcare applications that have been made possible by these technical advances. Some perspectives for the future of this technology will also be presented that promise to bring about new applications of this imaging modality in clinical research and healthcare.
Keywords: PET instrumentation; PET/CT; PET/MR; clinical applications; microPET; positron emission tomography.
Figures



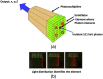
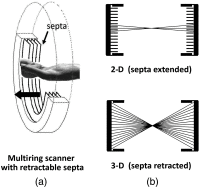


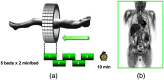
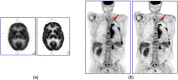



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

