Vinculin in cell-cell and cell-matrix adhesions
- PMID: 28401269
- PMCID: PMC5501900
- DOI: 10.1007/s00018-017-2511-3
Vinculin in cell-cell and cell-matrix adhesions
Abstract
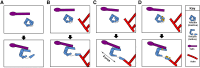
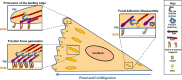
Vinculin was identified as a component of focal adhesions and adherens junctions nearly 40 years ago. Since that time, remarkable progress has been made in understanding its activation, regulation and function. Here we discuss the current understanding of the roles of vinculin in cell-cell and cell-matrix adhesions. Emphasis is placed on the how vinculin is recruited, activated and regulated. We also highlight the recent understanding of how vinculin responds to and transmits force at integrin- and cadherin-containing adhesion complexes to the cytoskeleton. Furthermore, we discuss roles of vinculin in binding to and rearranging the actin cytoskeleton.
Keywords: Cadherins; Cell adhesion; Cell migration; Force and mechanotransduction; Integrins; Vinculin.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

