Use of Flash Glucose-Sensing Technology for 12 months as a Replacement for Blood Glucose Monitoring in Insulin-treated Type 2 Diabetes
- PMID: 28401454
- PMCID: PMC5446381
- DOI: 10.1007/s13300-017-0255-6
Use of Flash Glucose-Sensing Technology for 12 months as a Replacement for Blood Glucose Monitoring in Insulin-treated Type 2 Diabetes
Abstract
Introduction: Published evaluations of sensor glucose monitoring use in insulin treated type 2 diabetes are limited. The aim of this study was to assess the impact of flash glucose-sensing technology as a replacement for self-monitoring of blood glucose (SMBG) over a 12-month period in participants with type 2 diabetes who were on intensive insulin therapy.
Methods: An open-label, randomized, controlled study in adults with type 2 diabetes on intensive insulin therapy from 26 European diabetes centers aimed at assessing flash glucose sensing technology was conducted. Participants (N = 224) were randomized (1:2 respectively) to a control group (n = 75) that used SMBG (FreeStyle Lite™) or to an intervention group (n = 149) which used sensor glucose data (FreeStyle Libre™ Flash Glucose Monitoring System) for self-management over 6 months. All intervention group participants who completed the 6-month treatment phase continued into an additional 6-month open-access phase.
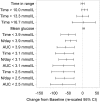
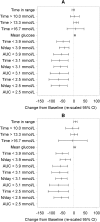
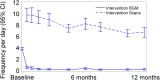
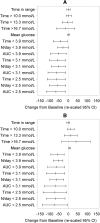
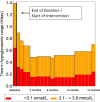
Results: A total of 139 intervention participants completed the 6-month treatment phase and continued into the open-access phase. At 12 months (end of open-access period), time in hypoglycemia [sensor glucose <3.9 mmol/L (70 mg/dL)] was reduced by 50% compared to baseline [-0.70 ± 1.85/24 h (mean ± standard deviation); p = 0.0002]. Nocturnal hypoglycemia [2300 to 0600 hours, <3.9 mmol/L (70 mg/dL)] was reduced by 52%; p = 0.0002. There was no change in time in range [sensor glucose 3.9-10.0 mmol/L (70-180 mg/dL)]. SMBG testing fell from a mean of 3.9 (median 3.9) times/day at baseline to 0.2 (0.0), with an average frequency of sensor scanning of 7.1 (5.7) times/day at 12 months, and mean sensor utilization was 83.6 ± 13.8% (median 88.3%) during the open-access phase. During this 6-month extension period no device-related serious adverse events were reported. Nine participants reported 16 instances of device-related adverse events (e.g. infection, allergy) and 28 participants (20.1%) experienced 134 occurrences of anticipated skin symptoms/sensor-insertion events expected with device use (e.g. erythema, itching and rash).
Conclusion: The use of flash glucose-sensing technology for glycemic management in individuals with type 2 diabetes treated by intensive insulin therapy over 12 months was associated with a sustained reduction in hypoglycemia and safely and effectively replaced SMBG.
Trial registration: ClinicalTrials.gov identifier, NCT02082184.
Keywords: Flash sensor glucose technology; Glucose monitoring; Insulin; Type 2 diabetes.
Figures
References
-
- American Diabetes Association Standards of medical care in diabetes 2017. Diabetes Care. 2017;40(Supple 1):S52.
-
- Khunti K, Alsifri S, Aronson R, Cigrovski Berkovic M, Enters-Weijnen C, Forsen T, Galstyan G, Geelhoed-Duijvestijn P, Goldfracht M, Gydesen H, Kapur R, Lalic N, Ludvik B, Moberg E, Pedersen-Bjergaard U, Ramchandran A (the HAT Investigator Group) Rates and predictors of hypoglycemia in 27,585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907–915. doi: 10.1111/dom.12689. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

