DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age
- PMID: 28401730
- PMCID: PMC5506444
- DOI: 10.1111/acel.12597
DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age
Abstract
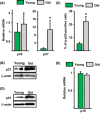
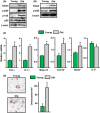
Age-related bone loss in mice results from a decrease in bone formation and an increase in cortical bone resorption. The former is accounted by a decrease in the number of postmitotic osteoblasts which synthesize the bone matrix and is thought to be the consequence of age-dependent changes in mesenchymal osteoblast progenitors. However, there are no specific markers for these progenitors, and conclusions rely on results from in vitro cultures of mixed cell populations. Moreover, the culprits of such changes remain unknown. Here, we have used Osx1-Cre;TdRFP mice in which osteoprogenitors express the TdRFP fluorescent protein. We report that the number of TdRFP-Osx1 cells, freshly isolated from the bone marrow, declines by more than 50% between 6 and 24 months of age in both female and male mice. Moreover, TdRFP-Osx1 cells from old mice exhibited markers of DNA damage and senescence, such as γH2AX foci, G1 cell cycle arrest, phosphorylation of p53, increased p21CIP1 levels, as well as increased levels of GATA4 and activation of NF-κB - two major stimulators of the senescence-associated secretory phenotype (SASP). Bone marrow stromal cells from old mice also exhibited elevated expression of SASP genes, including several pro-osteoclastogenic cytokines, and increased capacity to support osteoclast formation. These changes were greatly attenuated by the senolytic drug ABT263. Together, these findings suggest that the decline in bone mass with age is the result of intrinsic defects in osteoprogenitor cells, leading to decreased osteoblast numbers and increased support of osteoclast formation.
Keywords: ABT263; GATA4; NF-κB; osteoblasts; osteoporosis; p21; p53.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures





References
-
- Almeida M, Han L, Martin‐Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC (2007) Skeletal involution by age‐associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 282, 27285–27297. - PMC - PubMed
-
- Bellosta P, Masramon L, Mansukhani A, Basilico C (2003) p21(WAF1/CIP1) acts as a brake in osteoblast differentiation. J. Bone Miner. Res. 18, 818–826. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

