The Impact of Gene Polymorphisms on Anticoagulation Control With Warfarin
- PMID: 28401802
- PMCID: PMC6714698
- DOI: 10.1177/1076029617703483
The Impact of Gene Polymorphisms on Anticoagulation Control With Warfarin
Abstract
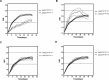
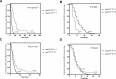
Differences in warfarin maintenance dosages based on the presence of polymorphisms in VKORC1, CYP2C9, CYP4F2, and ORM1 can be determined through dosage adjustment according to routine guidelines. Little is known about whether routine therapy could provide consensus anticoagulation control for patients with different genotypes. This study was carried out to compare anticoagulant control in patients with different genotypes. Six hundred seventy patients using warfarin according to Chinese guidelines were enrolled. Warfarin dosages and monitored international normalized ratios (INRs) were recorded. Genotypes of VKORC1 rs9923231, CYP4F2 rs2108622, CYP2C9 rs1057910, and ORM1 rs17650 polymorphisms were determined. Warfarin dosages and INR were compared between genotypes. Patients with the AGCC*F*F*1*1 polymorphism took longer than patients with the AACC*F*F*1*1 polymorphism (20 vs 5 days, P < .001) to achieve the targeted INR range. The INR values of patients with AACC*F*F*1*3 were unstable and did not enter the stable state control phase until after 35 days. The peak INR of patients with the AACC*F*F*1*3 polymorphism was exceedingly high, with some values exceeding the control range limit of 3.0. Patients with the AACC*F*S*1*1 or AACT*F*F*1*1 polymorphisms exhibited similar INR values as the patients with the AACC*F*F*1*1 polymorphism. This study found that routine medication with warfarin provides significantly different levels of anticoagulant control between patients with wild-type genotypes and patients with heterozygous polymorphism genotypes of VKORC1 rs9923231 or CYP2C9 rs1057910. Patients with heterozygous polymorphism genotypes of VKORC1 or CYP2C9 require genotype-directed therapy with warfarin to increase efficacy and safety in anticoagulant treatment.
Keywords: CYP2C9; CYP4F2; ORM1; VKORC1; warfarin.
Conflict of interest statement
Figures

References
-
- Lesko LJ. The critical path of warfarin dosing: finding an optimal dosing strategy using pharmacogenetics. Clin Pharmacol Ther. 2008;84(3):301–303. doi:10.1038/clpt.2008.133. - PubMed
-
- Lal S, Jada SR, Xiang X, Lim WT, Lee EJ, Chowbay B. Pharmacogenetics of target genes across the warfarin pharmacological pathway. Clin Pharmacokinet. 2006;45(12):1189–1200. - PubMed
-
- Garcia DA, Hylek E. Warfarin pharmacogenetics. N Engl J Med. 2009;360(23):2474; author reply 2475. doi:10.1056/NEJMc090579. - PubMed
-
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167(13):1414–1419. - PubMed
-
- Gage BF, Lesko LJ. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis. 2008;25(1):45–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

