Anisotropic forces from spatially constrained focal adhesions mediate contact guidance directed cell migration
- PMID: 28401884
- PMCID: PMC5394287
- DOI: 10.1038/ncomms14923
Anisotropic forces from spatially constrained focal adhesions mediate contact guidance directed cell migration
Abstract
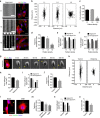
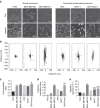
Directed migration by contact guidance is a poorly understood yet vital phenomenon, particularly for carcinoma cell invasion on aligned collagen fibres. We demonstrate that for single cells, aligned architectures providing contact guidance cues induce constrained focal adhesion maturation and associated F-actin alignment, consequently orchestrating anisotropic traction stresses that drive cell orientation and directional migration. Consistent with this understanding, relaxing spatial constraints to adhesion maturation either through reduction in substrate alignment density or reduction in adhesion size diminishes the contact guidance response. While such interactions allow single mesenchymal-like cells to spontaneously 'sense' and follow topographic alignment, intercellular interactions within epithelial clusters temper anisotropic cell-substratum forces, resulting in substantially lower directional response. Overall, these results point to the control of contact guidance by a balance of cell-substratum and cell-cell interactions, modulated by cell phenotype-specific cytoskeletal arrangements. Thus, our findings elucidate how phenotypically diverse cells perceive ECM alignment at the molecular level.
Conflict of interest statement
A.R., O.L., Z.W., R.M.E., P.W.A. and P.P.P. declare no competing financial interests. D.-H.K. is a co-founder and scientific board member of a start-up company, NanoSurface Biomedical that aims to commercialize nanopatterned polymeric cultureware.
Figures





References
-
- Rose D. M., Alon R. & Ginsberg M. H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 218, 126–134 (2007). - PubMed
-
- Yamaguchi H., Wyckoff J. & Condeelis J. Cell migration in tumors. Curr. Opin. Cell Biol. 17, 559–564 (2005). - PubMed
-
- Friedl P. & Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

