Iron Isotope Fractionation during Fe(II) Oxidation Mediated by the Oxygen-Producing Marine Cyanobacterium Synechococcus PCC 7002
- PMID: 28402123
- PMCID: PMC5415872
- DOI: 10.1021/acs.est.6b05833
Iron Isotope Fractionation during Fe(II) Oxidation Mediated by the Oxygen-Producing Marine Cyanobacterium Synechococcus PCC 7002
Abstract
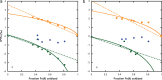
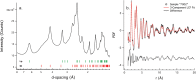
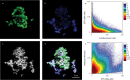
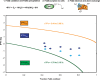
In this study, we couple iron isotope analysis to microscopic and mineralogical investigation of iron speciation during circumneutral Fe(II) oxidation and Fe(III) precipitation with photosynthetically produced oxygen. In the presence of the cyanobacterium Synechococcus PCC 7002, aqueous Fe(II) (Fe(II)aq) is oxidized and precipitated as amorphous Fe(III) oxyhydroxide minerals (iron precipitates, Feppt), with distinct isotopic fractionation (ε56Fe) values determined from fitting the δ56Fe(II)aq (1.79‰ and 2.15‰) and the δ56Feppt (2.44‰ and 2.98‰) data trends from two replicate experiments. Additional Fe(II) and Fe(III) phases were detected using microscopy and chemical extractions and likely represent Fe(II) and Fe(III) sorbed to minerals and cells. The iron desorbed with sodium acetate (FeNaAc) yielded heavier δ56Fe compositions than Fe(II)aq. Modeling of the fractionation during Fe(III) sorption to cells and Fe(II) sorption to Feppt, combined with equilibration of sorbed iron and with Fe(II)aq using published fractionation factors, is consistent with our resulting δ56FeNaAc. The δ56Feppt data trend is inconsistent with complete equilibrium exchange with Fe(II)aq. Because of this and our detection of microbially excreted organics (e.g., exopolysaccharides) coating Feppt in our microscopic analysis, we suggest that electron and atom exchange is partially suppressed in this system by biologically produced organics. These results indicate that cyanobacteria influence the fate and composition of iron in sunlit environments via their role in Fe(II) oxidation through O2 production, the capacity of their cell surfaces to sorb iron, and the interaction of secreted organics with Fe(III) minerals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Trouwborst R. E.; Johnston A.; Koch G.; Luther G. W. III; Pierson B. K. Biogeochemistry of Fe(II) oxidation in a photosynthetic microbial mat: Implications for Precambrian Fe(II) oxidation. Geochim. Cosmochim. Acta 2007, 71, 4629–4643. 10.1016/j.gca.2007.07.018. - DOI
-
- Epping E. H. G.; Schoemann V.; de Heij H. Manganese and Iron Oxidation During Benthic Oxygenic Photosynthesis. Estuarine, Coastal Shelf Sci. 1998, 47 (6), 753–767. 10.1006/ecss.1998.0401. - DOI
-
- Scholz F.; McManus J.; Mix A. C.; Hensen C.; Schneider R. R. The impact of ocean deoxygenation on iron release from continental margin sediments. Nat. Geosci. 2014, 7 (6), 433–437. 10.1038/ngeo2162. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

