Gut Microbial Diversity in Antibiotic-Naive Children After Systemic Antibiotic Exposure: A Randomized Controlled Trial
- PMID: 28402408
- PMCID: PMC5849050
- DOI: 10.1093/cid/cix141
Gut Microbial Diversity in Antibiotic-Naive Children After Systemic Antibiotic Exposure: A Randomized Controlled Trial
Abstract
Background: Antibiotic exposure can alter the gut microbiome. We evaluate the effects of azithromycin on the gut microbiome diversity of children from an antibiotic-naive community in Niger.
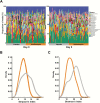
Methods: A population-based sample of 80 children aged 1-60 months in the Dosso region of Niger was randomized to receive a single dose of either oral azithromycin or placebo. Fecal samples were collected immediately before treatment and 5 days after treatment for 16S rRNA gene sequencing. The prespecified outcome was α-diversity (inverse Simpson's α-diversity index), with secondary outcomes of β and γ Simpson's and Shannon's diversities.
Results: At 5 days after treatment, 40 children aged 1-60 months were analyzed in the azithromycin-treated group and 40 children in the placebo-treated group. Diversity of the gut microbiome was significantly lower in the treated group (inverse Simpson's α-diversity, 5.03; 95% confidence interval [CI], 4.08-6.14) than in the placebo group (6.91; 95% CI, 5.82-8.21; P = .03). Similarly, the Shannon's α-diversity was lower in the treated group (10.60; 95% CI, 8.82-12.36) than the placebo group (15.42; 95% CI, 13.24-17.80; P = .004). Simpson's community-level (γ) diversity decreased with azithromycin exposure from 17.72 (95% CI, 13.80-20.21) to 10.10 (95% CI, 7.80-11.40; P = .00008), although β-diversity was not significantly reduced (2.56, 95% CI, 1.88-3.12; to 2.01, 95% CI, 1.46-2.51; P = .26).
Conclusions: Oral administration of azithromycin definitively decreases the diversity of the gut microbiome of children in an antibiotic-naive community.
Clinical trials registration: NCT02048007.
Keywords: antibiotics.; azithromycin; children; gut microbiome; randomized controlled trial.
Published by Oxford University Press for the Infectious Diseases Society of America 2017. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

