From the Cover: ImpairedProliferation and Differentiation of the Conducting Airway Epithelium Associated With Bronchiolitis Obliterans After Sulfur Mustard Inhalation Injury in Rats
- PMID: 28402575
- PMCID: PMC6075598
- DOI: 10.1093/toxsci/kfx057
From the Cover: ImpairedProliferation and Differentiation of the Conducting Airway Epithelium Associated With Bronchiolitis Obliterans After Sulfur Mustard Inhalation Injury in Rats
Abstract
Sulfur mustard (SM) is a chemical warfare agent that causes chronic airway remodeling. This study's objective was to assess for changes to the bronchiolar epithelium after SM exposure to explain its contribution to chronic airway remodeling.
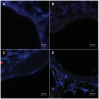
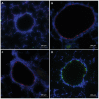
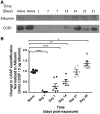
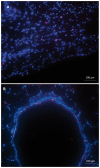
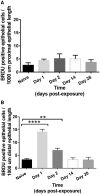
Materials and methods: Adult male rats were exposed to a sublethal dose of SM inhalation (1.0-1.2 mg/kg) for 50 min. Histological sections of the bronchiolar epithelium were analyzed for changes using hematoxylin and eosin, trichrome, and immunofluorescent staining for acetylated tubulin (AT) and club cell secretory protein (CCSP). CCSP in bronchoalveolar lavage fluid was assessed using western blot. A bromodeoxyuridine (BRDU) assay was used to assess for epithelial proliferation, and real-time PCR measured changes in Notch mRNA expression.
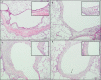
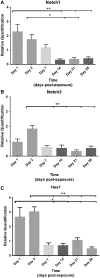
Results: SM caused significant proximal bronchiolar epithelial injury with epithelial denudation, loss of acetylated tubulin and CCSP staining, and reduced bronchoalveolar lavage fluid CCSP levels. bromodeoxyuridine (BRDU) + staining of proximal bronchiolar epithelial cells was not increased, but staining was increased in the distal bronchiolar epithelium. One month after injury, the proximal bronchiolar epithelium was not fully repaired. Significant collagen deposition surrounded proximal bronchioles with luminal obstruction, consistent with bronchiolitis obliterans. These changes corresponded with a downregulation of Notch1, Notch3, and Hes1 mRNA expressions.
Conclusions: This study demonstrates that SM exposure resulted in severe proximal airway epithelial injury, persistent morphological changes, impaired epithelial proliferation and, ultimately, bronchiolitis obliterans. These changes occurred at the same time that the Notch signaling genes were downregulated. Thus, the lung epithelium and the Notch signaling pathway may be worthy targets for the prevention of chronic airway remodeling after SM inhalation injury.
Keywords: Notch; bronchiolitis obliterans; epithelium; proliferation; regeneration; sulfur mustard.
Published by Oxford University Press on behalf of the Society of Toxicology 2017. This work is written by US Government employees and is in the public domain in the US.
Figures

References
-
- Allon N., Amir A., Manisterski E., Rabinovitz I., Dachir S., Kadar T. (2009). Inhalation exposure to sulfur mustard in the guinea pig model: Clinical, biochemical and histopathological characterization of respiratory injuries. Toxicol. Appl. Pharmacol. 241, 154–162. - PubMed
-
- Anderson DR, Byers SL, Vesely KR. 2000. Treatment of sulfur mustard (HD)-induced lung injury. J Appl Toxicol. 20 Suppl 1, S129–S132. - PubMed
-
- Beheshti J., Mark E. J., Akbaei H. M., Aslani J., Ghanei M. (2006). Mustard lung secrets: Long term clinicopathological study following mustard gas exposure. Pathol. Res. Pract. 202, 739–744. - PubMed
-
- Cabiati M., Raucci S., Caselli C., Guzzardi M. A., D’Amico A., Prescimone T., Del Ry S. (2012). Tissue-specific selection of stable reference genes for real-time PCR normalization in an obese rat model. J. Mol. Endocrinol. 48, 251–260. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

