Interdigitation between Triglycerides and Lipids Modulates Surface Properties of Lipid Droplets
- PMID: 28402884
- PMCID: PMC5390054
- DOI: 10.1016/j.bpj.2017.02.032
Interdigitation between Triglycerides and Lipids Modulates Surface Properties of Lipid Droplets
Abstract
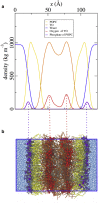
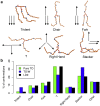
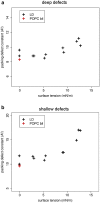
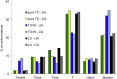
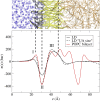
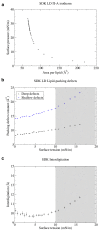
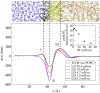
Intracellular lipid droplets (LDs) are the main cellular site of metabolic energy storage. Their structure is unique inside the cell, with a core of esterified fatty acids and sterols, mainly triglycerides and sterol esters, surrounded by a single monolayer of phospholipids. Numerous peripheral proteins, including several that were previously associated with intracellular compartments surrounded by a lipid bilayer, have been recently shown to target the surface of LDs, but how they are able to selectively target this organelle remains largely unknown. Here, we use atomistic and coarse-grained molecular dynamics simulations to investigate the molecular properties of the LD surface and to characterize how it differs from that of a lipid bilayer. Our data suggest that although several surface properties are remarkably similar between the two structures, key differences originate from the interdigitation between surface phospholipids and core neutral lipids that occurs in LDs. This property is extremely sensitive to membrane undulations, unlike in lipid bilayers, and it strongly affects both lipid-packing defects and the lateral pressure profile. We observed a marked change in overall surface properties for surface tensions >10 mN/m, indicative of a bimodal behavior. Our simulations provide a comprehensive molecular characterization of the unique surface properties of LDs and suggest how the molecular properties of the surface lipid monolayer can be modulated by the underlying neutral lipids.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

